Single-cell intracellular pH dynamics regulate the cell cycle by timing the G1 exit and G2 transition
- PMID: 37133398
- PMCID: PMC10281514
- DOI: 10.1242/jcs.260458
Single-cell intracellular pH dynamics regulate the cell cycle by timing the G1 exit and G2 transition
Abstract
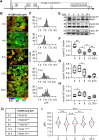
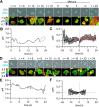
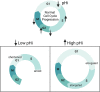
Transient changes in intracellular pH (pHi) regulate normal cell behaviors, but roles for spatiotemporal pHi dynamics in single-cell behaviors remain unclear. Here, we mapped single-cell spatiotemporal pHi dynamics during mammalian cell cycle progression both with and without cell cycle synchronization. We found that single-cell pHi is dynamic throughout the cell cycle: pHi decreases at G1/S, increases in mid-S, decreases at late S, increases at G2/M and rapidly decreases during mitosis. Importantly, although pHi is highly dynamic in dividing cells, non-dividing cells have attenuated pHi dynamics. Using two independent pHi manipulation methods, we found that low pHi inhibits completion of S phase whereas high pHi promotes both S/G2 and G2/M transitions. Our data also suggest that low pHi cues G1 exit, with decreased pHi shortening G1 and increased pHi elongating G1. Furthermore, dynamic pHi is required for S phase timing, as high pHi elongates S phase and low pHi inhibits S/G2 transition. This work reveals that spatiotemporal pHi dynamics are necessary for cell cycle progression at multiple phase transitions in single human cells.
Keywords: Cell cycle; Intracellular pH; Mitosis; Quantitative imaging; Single-cell methods; pH biosensor.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

