Colocalization of Chikungunya Virus with Its Receptor MXRA8 during Cell Attachment, Internalization, and Membrane Fusion
- PMID: 37133449
- PMCID: PMC10231136
- DOI: 10.1128/jvi.01557-22
Colocalization of Chikungunya Virus with Its Receptor MXRA8 during Cell Attachment, Internalization, and Membrane Fusion
Abstract
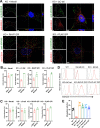
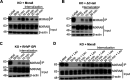
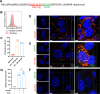
Arthritogenic alphaviruses, including chikungunya virus (CHIKV), preferentially target joint tissues and cause chronic rheumatic disease that adversely impacts the quality of life of patients. Viruses enter target cells via interaction with cell surface receptor(s), which determine the viral tissue tropism and pathogenesis. Although MXRA8 is a recently identified receptor for several clinically relevant arthritogenic alphaviruses, its detailed role in the cell entry process has not been fully explored. We found that in addition to its localization on the plasma membrane, MXRA8 is present in acidic organelles, endosomes, and lysosomes. Moreover, MXRA8 is internalized into cells without a requirement for its transmembrane and cytoplasmic domains. Confocal microscopy and live cell imaging revealed that MXRA8 interacts with CHIKV at the cell surface and then enters cells along with CHIKV particles. At the moment of membrane fusion in the endosomes, many viral particles are still colocalized with MXRA8. These findings provide insight as to how MXRA8 functions in alphavirus internalization and suggest possible targets for antiviral development. IMPORTANCE The globally distributed arthritogenic alphaviruses have infected millions of humans and induce rheumatic disease, such as severe polyarthralgia/polyarthritis, for weeks to years. Alphaviruses infect target cells through receptor(s) followed by clathrin-mediated endocytosis. MXRA8 was recently identified as an entry receptor that shapes the tropism and pathogenesis for multiple arthritogenic alphaviruses, including chikungunya virus (CHIKV). Nonetheless, the exact functions of MXRA8 during the process of viral cell entry remain undetermined. Here, we have provided compelling evidence for MXRA8 as a bona fide entry receptor that mediates the uptake of alphavirus virions. Small molecules that disrupt MXRA8-dependent binding of alphaviruses or internalization steps could serve as a platform for unique classes of antiviral drugs.
Keywords: MXRA8; chikungunya virus; entry; receptor.
Conflict of interest statement
The authors declare a conflict of interest. M.S.D. is a consultant for Inbios, Vir Biotechnology, Senda Biosciences, Moderna, Ocugen, and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions.
Figures



References
-
- Kiwanuka N, Sanders EJ, Rwaguma EB, Kawamata J, Ssengooba FP, Najjemba R, Were WA, Lamunu M, Bagambisa G, Burkot TR, Dunster L, Lutwama JJ, Martin DA, Cropp CB, Karabatsos N, Lanciotti RS, Tsai TF, Campbell GL. 1999. O’nyong-nyong fever in south-central Uganda, 1996–1997: clinical features and validation of a clinical case definition for surveillance purposes. Clin Infect Dis 29:1243–1250. doi: 10.1086/313462. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

