Inhibition of ACSL4 Alleviates Parkinsonism Phenotypes by Reduction of Lipid Reactive Oxygen Species
- PMID: 37133631
- PMCID: PMC10457271
- DOI: 10.1007/s13311-023-01382-4
Inhibition of ACSL4 Alleviates Parkinsonism Phenotypes by Reduction of Lipid Reactive Oxygen Species
Abstract
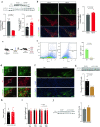
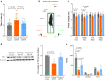
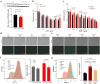
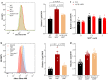
Ferroptosis is a programmed cell death pathway that is recently linked to Parkinson's disease (PD), where the key genes and molecules involved are still yet to be defined. Acyl-CoA synthetase long-chain family member 4 (ACSL4) esterifies polyunsaturated fatty acids (PUFAs) which is essential to trigger ferroptosis, and is suggested as a key gene in the pathogenesis of several neurological diseases including ischemic stroke and multiple sclerosis. Here, we report that ACSL4 expression in the substantia nigra (SN) was increased in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated model of PD and in dopaminergic neurons in PD patients. Knockdown of ACSL4 in the SN protected against dopaminergic neuronal death and motor deficits in the MPTP mice, while inhibition of ACSL4 activity with Triacsin C similarly ameliorated the parkinsonism phenotypes. Similar effects of ACSL4 reduction were observed in cells treated with 1-methyl-4-phenylpyridinium (MPP+) and it specifically prevented the lipid ROS elevation without affecting the mitochondrial ROS changes. These data support ACSL4 as a therapeutic target associated with lipid peroxidation in PD.
Keywords: ACSL4; Ferroptosis; Lipid peroxidation; Neuroprotection; Parkinson’s disease.
© 2023. The American Society for Experimental Neurotherapeutics, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Exosome-Derived CDC42 From Hypoxia-Pretreated Neural Stem Cells Inhibits ACSL4-Related Ferroptosis to Alleviate Vascular Injury in Parkinson's Disease Mice Models.J Neurochem. 2025 Mar;169(3):e70027. doi: 10.1111/jnc.70027. J Neurochem. 2025. PMID: 40035385
-
Ndfip1 protected dopaminergic neurons via regulating mitochondrial function and ferroptosis in Parkinson's disease.Exp Neurol. 2024 May;375:114724. doi: 10.1016/j.expneurol.2024.114724. Epub 2024 Feb 15. Exp Neurol. 2024. PMID: 38365133
-
Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson's Disease by Inhibiting Ferroptosis.Oxid Med Cell Longev. 2022 Sep 5;2022:7769355. doi: 10.1155/2022/7769355. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36105483 Free PMC article.
-
The role of ACSL4 in stroke: mechanisms and potential therapeutic target.Mol Cell Biochem. 2025 Apr;480(4):2223-2246. doi: 10.1007/s11010-024-05150-6. Epub 2024 Nov 4. Mol Cell Biochem. 2025. PMID: 39496916 Free PMC article. Review.
-
Oxidative stress and regulated cell death in Parkinson's disease.Ageing Res Rev. 2021 May;67:101263. doi: 10.1016/j.arr.2021.101263. Epub 2021 Feb 1. Ageing Res Rev. 2021. PMID: 33540042 Review.
Cited by
-
Lipid metabolism in health and disease: Mechanistic and therapeutic insights for Parkinson's disease.Chin Med J (Engl). 2025 Jun 20;138(12):1411-1423. doi: 10.1097/CM9.0000000000003627. Epub 2025 May 26. Chin Med J (Engl). 2025. PMID: 40419446 Free PMC article. Review.
-
DNALI1 Promotes Neurodegeneration after Traumatic Brain Injury via Inhibition of Autophagosome-Lysosome Fusion.Adv Sci (Weinh). 2024 Apr;11(15):e2306399. doi: 10.1002/advs.202306399. Epub 2024 Feb 13. Adv Sci (Weinh). 2024. PMID: 38348540 Free PMC article.
-
Energy metabolism in health and diseases.Signal Transduct Target Ther. 2025 Feb 18;10(1):69. doi: 10.1038/s41392-025-02141-x. Signal Transduct Target Ther. 2025. PMID: 39966374 Free PMC article. Review.
-
Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects.Signal Transduct Target Ther. 2024 Oct 14;9(1):271. doi: 10.1038/s41392-024-01969-z. Signal Transduct Target Ther. 2024. PMID: 39396974 Free PMC article. Review.
-
Mapping the Research of Ferroptosis in Parkinson's Disease from 2013 to 2023: A Scientometric Review.Drug Des Devel Ther. 2024 Apr 3;18:1053-1081. doi: 10.2147/DDDT.S458026. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38585257 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

