Systematic Review of the Psychometric Performance of Generic Childhood Multi-attribute Utility Instruments
- PMID: 37133712
- PMCID: PMC10232649
- DOI: 10.1007/s40258-023-00806-8
Systematic Review of the Psychometric Performance of Generic Childhood Multi-attribute Utility Instruments
Abstract
Background: Childhood multi-attribute utility instruments (MAUIs) can be used to measure health utilities in children (aged ≤ 18 years) for economic evaluation. Systematic review methods can generate a psychometric evidence base that informs their selection for application. Previous reviews focused on limited sets of MAUIs and psychometric properties, and only on evidence from studies that directly aimed to conduct psychometric assessments.
Objective: This study aimed to conduct a systematic review of psychometric evidence for generic childhood MAUIs and to meet three objectives: (1) create a comprehensive catalogue of evaluated psychometric evidence; (2) identify psychometric evidence gaps; and (3) summarise the psychometric assessment methods and performance by property.
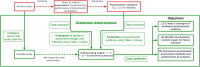
Methods: A review protocol was registered with the Prospective Register of Systematic Reviews (PROSPERO; CRD42021295959); reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline. The searches covered seven academic databases, and included studies that provided psychometric evidence for one or more of the following generic childhood MAUIs designed to be accompanied by a preference-based value set (any language version): 16D, 17D, AHUM, AQoL-6D, CH-6D, CHSCS-PS, CHU9D, EQ-5D-Y-3L, EQ-5D-Y-5L, HUI2, HUI3, IQI, QWB, and TANDI; used data derived from general and/or clinical childhood populations and from children and/or proxy respondents; and were published in English. The review included 'direct studies' that aimed to assess psychometric properties and 'indirect studies' that generated psychometric evidence without this explicit aim. Eighteen properties were evaluated using a four-part criteria rating developed from established standards in the literature. Data syntheses identified psychometric evidence gaps and summarised the psychometric assessment methods/results by property.
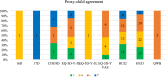
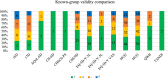
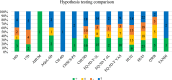
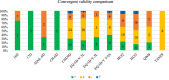
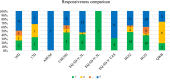
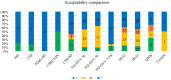
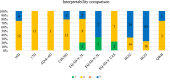
Results: Overall, 372 studies were included, generating a catalogue of 2153 criteria rating outputs across 14 instruments covering all properties except predictive validity. The number of outputs varied markedly by instrument and property, ranging from 1 for IQI to 623 for HUI3, and from zero for predictive validity to 500 for known-group validity. The more recently developed instruments targeting preschool children (CHSCS-PS, IQI, TANDI) have greater evidence gaps (lack of any evidence) than longer established instruments such as EQ-5D-Y, HUI2/3, and CHU9D. The gaps were prominent for reliability (test-retest, inter-proxy-rater, inter-modal, internal consistency) and proxy-child agreement. The inclusion of indirect studies (n = 209 studies; n = 900 outputs) increased the number of properties with at least one output of acceptable performance. Common methodological issues in psychometric assessment were identified, e.g., lack of reference measures to help interpret associations and changes. No instrument consistently outperformed others across all properties.
Conclusion: This review provides comprehensive evidence on the psychometric performance of generic childhood MAUIs. It assists analysts involved in cost-effectiveness-based evaluation to select instruments based on the application-specific minimum standards of scientific rigour. The identified evidence gaps and methodological issues also motivate and inform future psychometric studies and their methods, particularly those assessing reliability, proxy-child agreement, and MAUIs targeting preschool children.
© 2023. The Author(s).
Conflict of interest statement
Joseph Kwon, Sarah Smith, Rakhee Raghunandan, Martin Howell, Elisabeth Huynh, Sungwook Kim, Thomas Bentley, Nia Roberts, Emily Lancsar, Kirsten Howard, Germaine Wong, Jonathan Craig, and Stavros Petrou have no conflict of interest to declare.
Figures

Similar articles
-
Comparing the Psychometric Performance of Generic Paediatric Health-Related Quality of Life Instruments in Children and Adolescents with ADHD, Anxiety and/or Depression.Pharmacoeconomics. 2024 Jun;42(Suppl 1):57-77. doi: 10.1007/s40273-024-01354-2. Epub 2024 Feb 8. Pharmacoeconomics. 2024. PMID: 38329689 Free PMC article.
-
Psychometric Performance of Generic Childhood Multi-Attribute Utility Instruments in Preterm and Low Birthweight Populations: A Systematic Review.Children (Basel). 2023 Nov 10;10(11):1798. doi: 10.3390/children10111798. Children (Basel). 2023. PMID: 38002889 Free PMC article. Review.
-
Psychometric Properties of Generic Preference-Weighted Measures for Children and Adolescents: A Systematic Review.Pharmacoeconomics. 2023 Feb;41(2):155-174. doi: 10.1007/s40273-022-01205-y. Epub 2022 Nov 21. Pharmacoeconomics. 2023. PMID: 36404365
-
Comparative Psychometric Performance of Common Generic Paediatric Health-Related Quality of Life Instrument Descriptive Systems: Results from the Australian Paediatric Multi-Instrument Comparison Study.Pharmacoeconomics. 2024 Jun;42(Suppl 1):39-55. doi: 10.1007/s40273-023-01330-2. Epub 2023 Nov 13. Pharmacoeconomics. 2024. PMID: 37955799 Free PMC article.
-
A review of the psychometric properties of generic utility measures in multiple sclerosis.Pharmacoeconomics. 2014 Aug;32(8):759-73. doi: 10.1007/s40273-014-0167-5. Pharmacoeconomics. 2014. PMID: 24846760 Review.
Cited by
-
Psychometric Properties of Child Health Utility 9D (CHU9D) Proxy Version Administered to Parents and Caregivers of Children Aged 2-4 Years Compared with Pediatric Quality of Life Inventory™ (PedsQL).Pharmacoeconomics. 2024 Jun;42(Suppl 1):147-161. doi: 10.1007/s40273-024-01355-1. Epub 2024 Jan 27. Pharmacoeconomics. 2024. PMID: 38280126 Free PMC article.
-
"If we ask, we must act": co-designing the implementation of the EQ-5D-Y-5L as a Paediatric Patient Reported Outcome Measure in Routine hospital Outpatient Care for Kids to meaningfully impact clinical visits (P-PROM ROCK Phase 2).Qual Life Res. 2025 Aug;34(8):2205-2218. doi: 10.1007/s11136-025-03996-x. Epub 2025 May 31. Qual Life Res. 2025. PMID: 40448866 Free PMC article.
-
Meeting the Challenges of Preference-Weighted Health-Related Quality-of-Life Measurement in Children.Pharmacoeconomics. 2024 Jun;42(Suppl 1):3-8. doi: 10.1007/s40273-024-01383-x. Epub 2024 May 9. Pharmacoeconomics. 2024. PMID: 38722540 Free PMC article. No abstract available.
-
Comparing the Psychometric Performance of Generic Paediatric Health-Related Quality of Life Instruments in Children and Adolescents with ADHD, Anxiety and/or Depression.Pharmacoeconomics. 2024 Jun;42(Suppl 1):57-77. doi: 10.1007/s40273-024-01354-2. Epub 2024 Feb 8. Pharmacoeconomics. 2024. PMID: 38329689 Free PMC article.
-
Fighting to Breathe and Fighting for Health-Related Quality of Life: Measuring the Impact of Being Dependent on Technology for Breathing on the Child and Their Caregiver.Patient. 2024 Jan;17(1):65-82. doi: 10.1007/s40271-023-00657-4. Epub 2023 Nov 22. Patient. 2024. PMID: 37991685 Free PMC article.
References
-
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
-
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. PMG92013. - PubMed
-
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada 4th Edition. Canadian Agency for Drugs and Technologies in Health; 2017.
-
- Pharmaceutical Benefits Advisory Committee. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (Version 5). Pharmaceutical Benefits Advisory Committee; 2016.
-
- Scottish Medicines Consortium. Working with SMC—a guide for manufacturers. Scottish Medicines Consortium; 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

