Evaluation of Waning of SARS-CoV-2 Vaccine-Induced Immunity: A Systematic Review and Meta-analysis
- PMID: 37133863
- PMCID: PMC10157431
- DOI: 10.1001/jamanetworkopen.2023.10650
Evaluation of Waning of SARS-CoV-2 Vaccine-Induced Immunity: A Systematic Review and Meta-analysis
Abstract
Importance: Estimates of the rate of waning of vaccine effectiveness (VE) against COVID-19 are key to assess population levels of protection and future needs for booster doses to face the resurgence of epidemic waves.
Objective: To quantify the progressive waning of VE associated with the Delta and Omicron variants of SARS-CoV-2 by number of received doses.
Data sources: PubMed and Web of Science were searched from the databases' inception to October 19, 2022, as well as reference lists of eligible articles. Preprints were included.
Study selection: Selected studies for this systematic review and meta-analysis were original articles reporting estimates of VE over time against laboratory-confirmed SARS-CoV-2 infection and symptomatic disease.
Data extraction and synthesis: Estimates of VE at different time points from vaccination were retrieved from original studies. A secondary data analysis was performed to project VE at any time from last dose administration, improving the comparability across different studies and between the 2 considered variants. Pooled estimates were obtained from random-effects meta-analysis.
Main outcomes and measures: Outcomes were VE against laboratory-confirmed Omicron or Delta infection and symptomatic disease and half-life and waning rate associated with vaccine-induced protection.
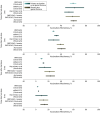
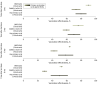
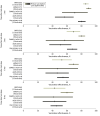
Results: A total of 799 original articles and 149 reviews published in peer-reviewed journals and 35 preprints were identified. Of these, 40 studies were included in the analysis. Pooled estimates of VE of a primary vaccination cycle against laboratory-confirmed Omicron infection and symptomatic disease were both lower than 20% at 6 months from last dose administration. Booster doses restored VE to levels comparable to those acquired soon after the administration of the primary cycle. However, 9 months after booster administration, VE against Omicron was lower than 30% against laboratory-confirmed infection and symptomatic disease. The half-life of VE against symptomatic infection was estimated to be 87 days (95% CI, 67-129 days) for Omicron compared with 316 days (95% CI, 240-470 days) for Delta. Similar waning rates of VE were found for different age segments of the population.
Conclusions and relevance: These findings suggest that the effectiveness of COVID-19 vaccines against laboratory-confirmed Omicron or Delta infection and symptomatic disease rapidly wanes over time after the primary vaccination cycle and booster dose. These results can inform the design of appropriate targets and timing for future vaccination programs.
Conflict of interest statement
Figures

References
-
- Wu N, Joyal-Desmarais K, Ribeiro PAB, et al. . Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med. Published online February 10, 2023. doi:10.1016/S2213-2600(23)00015-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

