γδ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer
- PMID: 37134157
- PMCID: PMC10156127
- DOI: 10.1126/sciadv.adf0108
γδ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer
Abstract
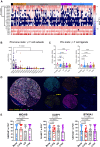
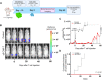
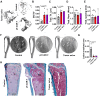
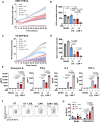
Immune checkpoint blockade has been largely unsuccessful for the treatment of bone metastatic castrate-resistant prostate cancer (mCRPC). Here, we report a combinatorial strategy to treat mCRPC using γδ-enriched chimeric antigen receptor (CAR) T cells and zoledronate (ZOL). In a preclinical murine model of bone mCRPC, γδ CAR-T cells targeting prostate stem cell antigen (PSCA) induced a rapid and significant regression of established tumors, combined with increased survival and reduced cancer-associated bone disease. Pretreatment with ZOL, a U.S. Food and Drug Administration-approved bisphosphonate prescribed to mitigate pathological fracture in mCRPC patients, resulted in CAR-independent activation of γδ CAR-T cells, increased cytokine secretion, and enhanced antitumor efficacy. These data show that the activity of the endogenous Vγ9Vδ2 T cell receptor is preserved in CAR-T cells, allowing for dual-receptor recognition of tumor cells. Collectively, our findings support the use of γδ CAR-T cell therapy for mCRPC treatment.
Figures



References
-
- Bubendorf L., Schopfer A., Wagner U., Sauter G., Moch H., Willi N., Gasser T. C., Mihatsch M. J., Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 31, 578–583 (2000). - PubMed
-
- Arriaga J. M., Panja S., Alshalalfa M., Zhao J., Zou M., Giacobbe A., Madubata C. J., Kim J. Y., Rodriguez A., Coleman I., Virk R. K., Hibshoosh H., Ertunc O., Ozbek B., Fountain J., Jeffrey Karnes R., Luo J., Antonarakis E. S., Nelson P. S., Feng F. Y., Rubin M. A., De Marzo A. M., Rabadan R., Sims P. A., Mitrofanova A., Abate-Shen C., A MYC and RAS co-activation signature in localized prostate cancer drives bone metastasis and castration resistance. Cancer 1, 1082–1096 (2020). - PMC - PubMed
-
- Greenspan S. L., Coates P., Sereika S. M., Nelson J. B., Trump D. L., Resnick N. M., Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J. Clin. Endocrinol. Metab. 90, 6410–6417 (2005). - PubMed
-
- Campbell S. C., Bhoopalam N., Moritz T. E., Pandya M., Iyer P., Vanveldhuizen P., Ellis N. K., Thottapurathu L., Garewal H., Warren S. R., Friedman N., Reda D. J., The use of zoledronic acid in men receiving androgen deprivation therapy for prostate cancer with severe osteopenia or osteoporosis. Urology 75, 1138–1143 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

