Design, Synthesis, Biological Evaluation, and Crystallographic Study of Novel Purine Nucleoside Phosphorylase Inhibitors
- PMID: 37134237
- PMCID: PMC10226123
- DOI: 10.1021/acs.jmedchem.2c02097
Design, Synthesis, Biological Evaluation, and Crystallographic Study of Novel Purine Nucleoside Phosphorylase Inhibitors
Abstract
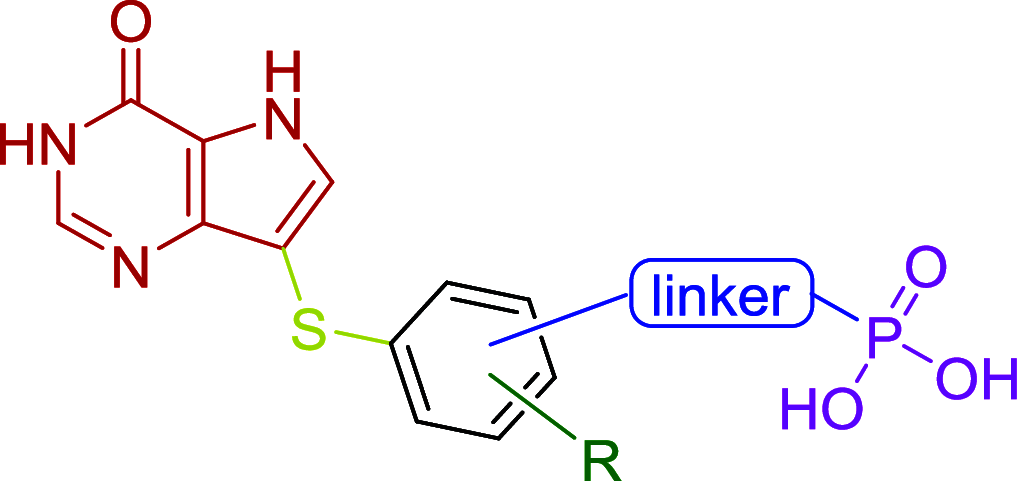
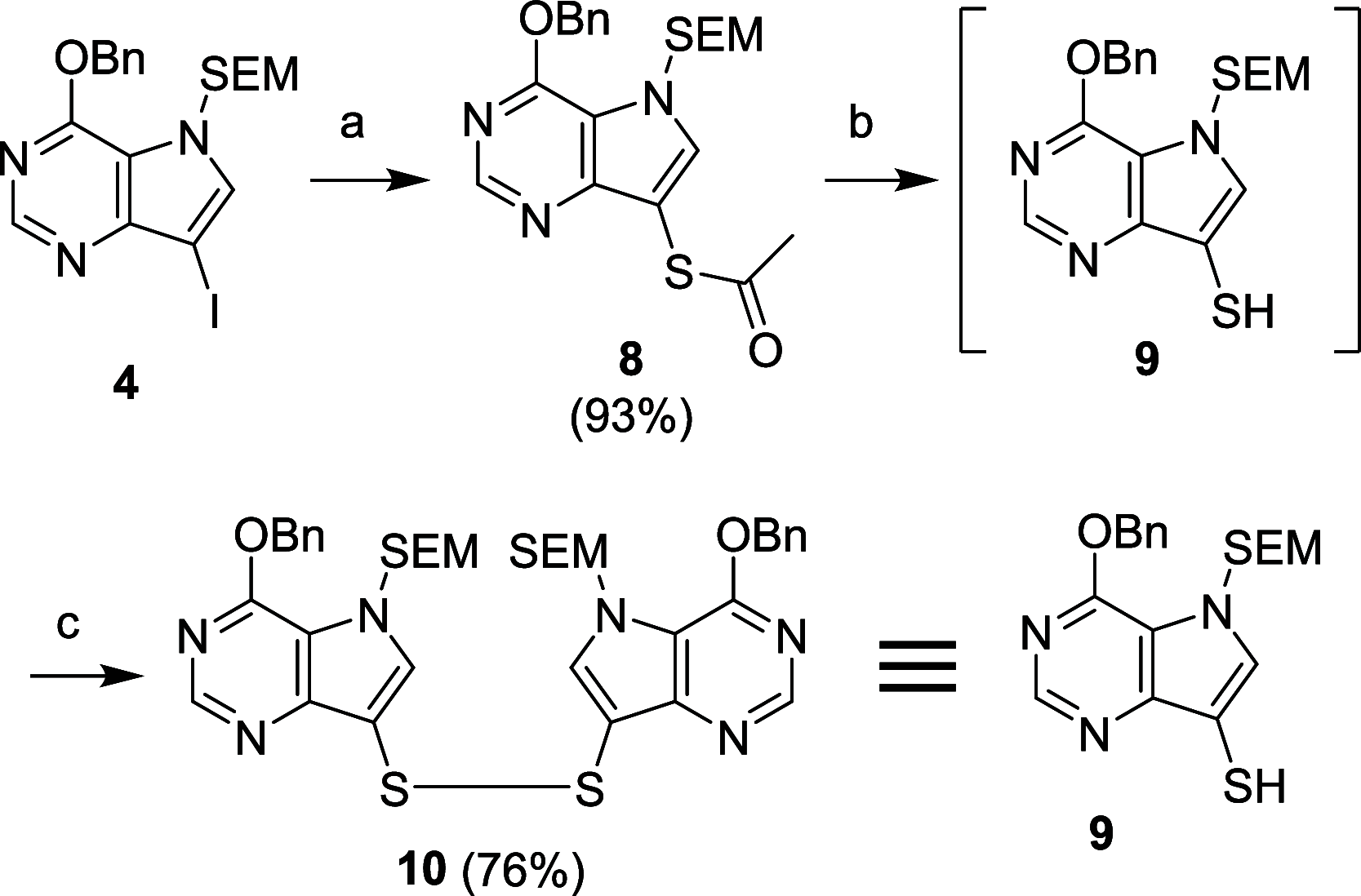
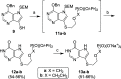
Purine nucleoside phosphorylase (PNP) is a well-known molecular target with potential therapeutic applications in the treatment of T-cell malignancies and/or bacterial/parasitic infections. Here, we report the design, development of synthetic methodology, and biological evaluation of a series of 30 novel PNP inhibitors based on acyclic nucleoside phosphonates bearing a 9-deazahypoxanthine nucleobase. The strongest inhibitors exhibited IC50 values as low as 19 nM (human PNP) and 4 nM (Mycobacterium tuberculosis (Mt) PNP) and highly selective cytotoxicity toward various T-lymphoblastic cell lines with CC50 values as low as 9 nM. No cytotoxic effect was observed on other cancer cell lines (HeLa S3, HL60, HepG2) or primary PBMCs for up to 10 μM. We report the first example of the PNP inhibitor exhibiting over 60-fold selectivity for the pathogenic enzyme (MtPNP) over hPNP. The results are supported by a crystallographic study of eight enzyme-inhibitor complexes and by ADMET profiling in vitro and in vivo.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
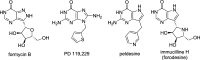















Similar articles
-
Development of an immobilized Mycobacterium tuberculosis purine nucleoside phosphorylase platform for ligand fishing and inhibition assays.J Pharm Biomed Anal. 2025 Feb 15;254:116576. doi: 10.1016/j.jpba.2024.116576. Epub 2024 Nov 22. J Pharm Biomed Anal. 2025. PMID: 39603195
-
New catalytic mechanism for human purine nucleoside phosphorylase.Biochem Biophys Res Commun. 2005 Feb 18;327(3):646-9. doi: 10.1016/j.bbrc.2004.12.052. Biochem Biophys Res Commun. 2005. PMID: 15649395
-
Purine nucleoside phosphorylase from Mycobacterium tuberculosis. Analysis of inhibition by a transition-state analogue and dissection by parts.Biochemistry. 2001 Jul 27;40(28):8196-203. doi: 10.1021/bi010584x. Biochemistry. 2001. PMID: 11444965
-
Perspectives and challenges in developing small molecules targeting purine nucleoside phosphorylase.Eur J Med Chem. 2024 May 5;271:116437. doi: 10.1016/j.ejmech.2024.116437. Epub 2024 Apr 20. Eur J Med Chem. 2024. PMID: 38701712 Review.
-
Transition states and inhibitors of the purine nucleoside phosphorylase family.Curr Top Med Chem. 2005;5(13):1237-58. doi: 10.2174/156802605774463088. Curr Top Med Chem. 2005. PMID: 16305529 Review.
Cited by
-
Purine nucleoside phosphorylase dominates Influenza A virus replication and host hyperinflammation through purine salvage.Signal Transduct Target Ther. 2025 Jun 15;10(1):191. doi: 10.1038/s41392-025-02272-1. Signal Transduct Target Ther. 2025. PMID: 40517177 Free PMC article.
References
-
- Basso L. A.; Santos D. S.; Shi W.; Furneaux R. H.; Tyler P. C.; Schramm V. L.; Blanchard J. S. Purine Nucleoside Phosphorylase from Mycobacterium Tuberculosis. Analysis of Inhibition by a Transition-State Analogue and Dissection by Parts. Biochemistry 2001, 40, 8196–8203. 10.1021/BI010584X. - DOI - PubMed
-
- Nolasco D. O.; Canduri F.; Pereira J. H.; Cortinóz J. R.; Palma M. S.; Oliveira J. S.; Basso L. A.; de Azevedo W. F.; Santos D. S. Crystallographic Structure of PNP from Mycobacterium Tuberculosis at 1.9 Å Resolution. Biochem. Biophys. Res. Commun. 2004, 324, 789–794. 10.1016/J.BBRC.2004.09.137. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

