Structure-based discovery of conformationally selective inhibitors of the serotonin transporter
- PMID: 37137306
- PMCID: PMC10306110
- DOI: 10.1016/j.cell.2023.04.010
Structure-based discovery of conformationally selective inhibitors of the serotonin transporter
Abstract
The serotonin transporter (SERT) removes synaptic serotonin and is the target of anti-depressant drugs. SERT adopts three conformations: outward-open, occluded, and inward-open. All known inhibitors target the outward-open state except ibogaine, which has unusual anti-depressant and substance-withdrawal effects, and stabilizes the inward-open conformation. Unfortunately, ibogaine's promiscuity and cardiotoxicity limit the understanding of inward-open state ligands. We docked over 200 million small molecules against the inward-open state of the SERT. Thirty-six top-ranking compounds were synthesized, and thirteen inhibited; further structure-based optimization led to the selection of two potent (low nanomolar) inhibitors. These stabilized an outward-closed state of the SERT with little activity against common off-targets. A cryo-EM structure of one of these bound to the SERT confirmed the predicted geometry. In mouse behavioral assays, both compounds had anxiolytic- and anti-depressant-like activity, with potencies up to 200-fold better than fluoxetine (Prozac), and one substantially reversed morphine withdrawal effects.
Keywords: depression; docking; functional selectivity; serotonin transporter; ultra-large libraries.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.K.S. serves on the SAB of Schrödinger, Umbra Therapeutics, and Deep Apple; with J.J.I., he co-founded Deep Apple Therapeutics and Blue Dolphin; with A.M. and B.L.R., he co-founded Epiodyne Ltd, while A.M. also founded Stipple Bio and consults for Abolone and Septerna. W.C.W. consults for Onsero Therapeutics, company co-founded by B.L.R., who also serves on the SAB of Escient Pharmaceuticals and of Septerna.
Figures

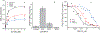


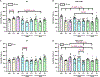


Comment in
-
Docking on SERT reveals new inhibitors.Nat Rev Drug Discov. 2023 Jul;22(7):536. doi: 10.1038/d41573-023-00089-7. Nat Rev Drug Discov. 2023. PMID: 37268852 No abstract available.
References
-
- Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, and Wendland JR (2008). How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology 55, 932–960. - PMC - PubMed
-
- Gu H, Wall SC, and Rudnick G (1994). Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem 269, 7124–7130. - PubMed
-
- Jacobs M, Zhang Y, Campbell S, and Rudnick G (2007). Ibogaine, a noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. J Biol Chem 282, 29441–29447. - PubMed
-
- Bulling S, Schicker K, Zhang Y-W, Stockner T, Gruber C, Boehm S, Freissmuth M, Rudnick G, Sitte HH, and Sandtner W (2012). The mechanistic basis of non-competitive ibogaine inhibition in serotonin and dopamine transporters. Journal of Biological Chemistry 287, 18524–18534. doi: 10.1074/jbc.M112.343681. - DOI - PMC - PubMed
-
- Glick SD, Maisonneuve IM, and Szumlinski KK (2001). Mechanisms of Action of Ibogaine: Relevance to Putative Therapeutic Effects and Development of a Safer Iboga Alkaloid Congener. In Ibogaine: Proceedings of the first International Conference, Alper KR, and Glick SD, eds. (Academic Press; ), pp. 39–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

