Speech- and language-linked FOXP2 mutation targets protein motors in striatal neurons
- PMID: 37137515
- PMCID: PMC10393416
- DOI: 10.1093/brain/awad090
Speech- and language-linked FOXP2 mutation targets protein motors in striatal neurons
Abstract
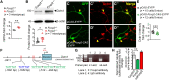
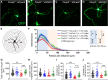
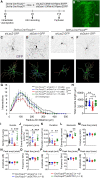
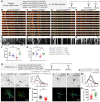
Human speech and language are among the most complex motor and cognitive abilities. The discovery of a mutation in the transcription factor FOXP2 in KE family members with speech disturbances has been a landmark example of the genetic control of vocal communication in humans. Cellular mechanisms underlying this control have remained unclear. By leveraging FOXP2 mutation/deletion mouse models, we found that the KE family FOXP2R553H mutation directly disables intracellular dynein-dynactin 'protein motors' in the striatum by induction of a disruptive high level of dynactin1 that impairs TrkB endosome trafficking, microtubule dynamics, dendritic outgrowth and electrophysiological activity in striatal neurons alongside vocalization deficits. Dynactin1 knockdown in mice carrying FOXP2R553H mutations rescued these cellular abnormalities and improved vocalization. We suggest that FOXP2 controls vocal circuit formation by regulating protein motor homeostasis in striatal neurons, and that its disruption could contribute to the pathophysiology of FOXP2 mutation/deletion-associated speech disorders.
Keywords: basal ganglia; endosome trafficking; microtubule; striatum; vocalization.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures




Similar articles
-
Mice carrying a humanized Foxp2 knock-in allele show region-specific shifts of striatal Foxp2 expression levels.Cortex. 2019 Sep;118:212-222. doi: 10.1016/j.cortex.2019.01.008. Epub 2019 Jan 30. Cortex. 2019. PMID: 30808549
-
Foxp2 controls synaptic wiring of corticostriatal circuits and vocal communication by opposing Mef2c.Nat Neurosci. 2016 Nov;19(11):1513-1522. doi: 10.1038/nn.4380. Epub 2016 Sep 5. Nat Neurosci. 2016. PMID: 27595386 Free PMC article.
-
Expression of FoxP2 in the basal ganglia regulates vocal motor sequences in the adult songbird.Nat Commun. 2021 May 11;12(1):2617. doi: 10.1038/s41467-021-22918-2. Nat Commun. 2021. PMID: 33976169 Free PMC article.
-
From songbird to humans: The multifaceted roles of FOXP2 in speech and motor learning.Neurosci Biobehav Rev. 2024 Dec;167:105936. doi: 10.1016/j.neubiorev.2024.105936. Epub 2024 Nov 6. Neurosci Biobehav Rev. 2024. PMID: 39510218 Review.
-
Singing mice, songbirds, and more: models for FOXP2 function and dysfunction in human speech and language.J Neurosci. 2006 Oct 11;26(41):10376-9. doi: 10.1523/JNEUROSCI.3379-06.2006. J Neurosci. 2006. PMID: 17035521 Free PMC article. Review.
Cited by
-
Compensation between FOXP transcription factors maintains proper striatal function.Cell Rep. 2024 May 28;43(5):114257. doi: 10.1016/j.celrep.2024.114257. Epub 2024 May 17. Cell Rep. 2024. PMID: 38761373 Free PMC article.
-
Modelling TDP-43 proteinopathy in Drosophila uncovers shared and neuron-specific targets across ALS and FTD relevant circuits.Acta Neuropathol Commun. 2023 Oct 20;11(1):168. doi: 10.1186/s40478-023-01656-0. Acta Neuropathol Commun. 2023. PMID: 37864255 Free PMC article.
-
Compensation between FOXP transcription factors maintains proper striatal function.bioRxiv [Preprint]. 2023 Jun 26:2023.06.26.546567. doi: 10.1101/2023.06.26.546567. bioRxiv. 2023. Update in: Cell Rep. 2024 May 28;43(5):114257. doi: 10.1016/j.celrep.2024.114257. PMID: 37425820 Free PMC article. Updated. Preprint.
-
Wings of Change: aPKC/FoxP-dependent plasticity in steering motor neurons underlies operant self-learning in Drosophila.F1000Res. 2024 Jun 11;13:116. doi: 10.12688/f1000research.146347.2. eCollection 2024. F1000Res. 2024. PMID: 38779314 Free PMC article.
-
Tubular FoxP2 and Kidney Fibrosis.J Am Soc Nephrol. 2025 Apr 1;36(4):544-558. doi: 10.1681/ASN.0000000576. Epub 2024 Dec 10. J Am Soc Nephrol. 2025. PMID: 39656554
References
-
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. - PubMed
-
- Reuter MS, Riess A, Moog U, et al. . FOXP2 Variants in 14 individuals with developmental speech and language disorders broaden the mutational and clinical spectrum. J Med Genet. 2017;54:64–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

