BCMA CAR-T induces complete and durable remission in refractory plasmablastic lymphoma
- PMID: 37137553
- PMCID: PMC10163502
- DOI: 10.1136/jitc-2023-006684
BCMA CAR-T induces complete and durable remission in refractory plasmablastic lymphoma
Abstract
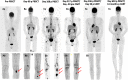
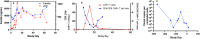
Plasmablastic lymphoma (PBL) is a rare subtype of aggressive large B-cell lymphoma, with a dismal prognosis despite aggressive therapies. New approaches are needed for those with refractory disease. PBL expresses antigens similar to multiple myeloma (MM), including B-cell maturation antigen (BCMA). Chimeric antigen receptor T-cell (CAR-T) therapy directed against BCMA has shown efficacy for the treatment of heavily pretreated MM with low rates of grades 3 and 4 cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) in a phase Ib/II trial (A Study of JNJ-68284528, a CAR-T Directed Against BCMA in Participants With Relapsed or Refractory Multiple Myeloma (CARTITUDE-1), NCT03548207). However, data for the use of BCMA CAR-T for treating PBL are lacking.We report a challenging case of multiple refractory PBL that emerged from B-cell acute lymphoblastic leukemia in an adolescent who failed to respond to an allogeneic hematopoietic cell transplant. The patient developed rapidly advancing disease despite withdrawal of immunosuppression, treatment with etoposide, ibrutinib, and daratumumab, prompting consideration of BCMA CAR-T (under emergency investigational new drug (eIND)). The patient achieved a complete remission (CR), without recurrent acute graft versus host disease (GVHD), CRS or ICANS after BCMA CAR-T therapy. BCMA CAR-T expansion was detected in vivo, peaking on day 15. The patient remains in CR for more than a year post CAR-T therapy, supporting consideration of immunotherapy for future patients with refractory PBL, a disease with few treatment options.
Keywords: Immunotherapy; Pediatrics; Receptors, Chimeric Antigen.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MQ reports honoraria from Vertex and Novartis. MVD is on the advisory board for Janssen, Sanofi, and Lava Therapeutics. SC is on the science advisory board of SOBI. JLK reports consulting for Abbvie, BMS, and DSnC for Incyte. JMS reports employment, equity and stock options at Janssen. DM reports employment at Janssen Research & Development, being adjunct assistant professor at Mount Sinai Hospital, Icahn School of Medicine, and being on the consultancy/advisory board at Takeda, Celgene, BMS, GlaxoSmithKline, Legend, Janssen, Kinevant, and Foundation Medicine. CCJ is an employee of Janssen Research & Development and physician consultant at Memorial Sloan Kettering Cancer Center. EZ is an employee of Janssen Research & Development. AT-M is an employee of Janssen Research & Development and an adjunct assistant professor at Los Angeles County/University of Southern California. AB is an employee of Janssen Research & Development. MV is an employee of Janssen-Cilag GmbH. TN reports being an employee of Caribou Biosciences, Inc., in Berkeley, California. NL reports being a full-time employee of Janssen. LP is an employee at Legend Biotech.
Figures
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
