Lipoprotein(a), Oxidized Phospholipids, and Coronary Artery Disease Severity and Outcomes
- PMID: 37137588
- PMCID: PMC10824318
- DOI: 10.1016/j.jacc.2023.02.050
Lipoprotein(a), Oxidized Phospholipids, and Coronary Artery Disease Severity and Outcomes
Abstract
Background: Lipoprotein(a) (Lp[a]) and oxidized phospholipids (OxPLs) are each independent risk factors for atherosclerotic cardiovascular disease. The extent to which Lp(a) and OxPLs predict coronary artery disease (CAD) severity and outcomes in a contemporary, statin-treated cohort is not well established.
Objectives: This study sought to evaluate the relationships between Lp(a) particle concentration and OxPLs associated with apolipoprotein B (OxPL-apoB) or apolipoprotein(a) (OxPL-apo[a]) with angiographic CAD and cardiovascular outcomes.
Methods: Among 1,098 participants referred for coronary angiography in the CASABLANCA (Catheter Sampled Blood Archive in Cardiovascular Diseases) study, Lp(a), OxPL-apoB, and OxPL-apo(a) were measured. Logistic regression estimated the risk of multivessel coronary stenoses by Lp(a)-related biomarker level. Cox proportional hazards regression estimated the risk of major adverse cardiovascular events (MACEs) (coronary revascularization, nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death) in follow-up.
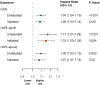
Results: Median Lp(a) was 26.45 nmol/L (IQR: 11.39-89.49 nmol/L). Lp(a), OxPL-apoB, and OxPL-apo(a) were highly correlated (Spearman R ≥0.91 for all pairwise combinations). Lp(a) and OxPL-apoB were associated with multivessel CAD. Odds of multivessel CAD per doubling of Lp(a), OxPL-apoB, and OxPL-apo(a) were 1.10 (95% CI: 1.03-1.18; P = 0.006), 1.18 (95% CI: 1.03-1.34; P = 0.01), and 1.07 (95% CI: 0.99-1.16; P = 0.07), respectively. All biomarkers were associated with cardiovascular events. HRs for MACE per doubling of Lp(a), OxPL-apoB, and OxPL-apo(a) were 1.08 (95% CI: 1.03-1.14; P = 0.001), 1.15 (95% CI: 1.05-1.26; P = 0.004), and 1.07 (95% CI: 1.01-1.14; P = 0.02), respectively.
Conclusions: In patients undergoing coronary angiography, Lp(a) and OxPL-apoB are associated with multivessel CAD. Lp(a), OxPL-apoB, and OxPL-apo(a) are associated with incident cardiovascular events. (Catheter Sampled Blood Archive in Cardiovascular Diseases [CASABLANCA]; NCT00842868).
Keywords: atherosclerosis; coronary artery disease; lipids and lipoproteins; lipoprotein(a).
Copyright © 2023 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This study was sponsored by Novartis. The sponsor provided feedback on the study design but not data interpretation. Dr Gilliland has received partial supported from the National Heart, Lung, and Blood Institute (NHLBI) (T32 grant 5T32HL125232). Dr Mohebi has received support from the Dennis and Marilyn Barry fellowship. Dr Hu is an employee of Novartis. Mr Cristino was an employee of Novartis at the time of manuscript preparation. Dr Browne is an employee of Novartis. Dr Tsimikas has received support from the NHLBI (grant R01HL159156), Fondation Leducq, and Novartis (research grant for the measurement of Lp[a] and OxPL levels); is a co-inventor and has received royalties from patents owned by the University of California San Diego (UCSD); is a co-founder and has an equity interest in Oxitope and its affiliates, Kleanthi Diagnostics, and Covicept Therapeutics; and has a dual appointment at UCSD and Ionis Pharmaceuticals. Dr Januzzi has received partial support from the Hutter Family Professorship; has served as a trustee of the American College of Cardiology; has served as a board member of Imbria Pharmaceuticals; has received grant support from Applied Therapeutics, Innolife, Novartis Pharmaceuticals, and Abbott Diagnostics; has received consulting income from Abbott, Janssen, Jana Care, Novartis, Prevencio, and Roche Diagnostics; and has participated in clinical endpoint committees or data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia, and Takeda. Dr Natarajan has received support from the NHLBI (grants R01HL142711, R01HL127564, R01HL148050, R01HL151283, R01HL148565, R01HL135242, and R01HL151152), the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01DK125782), Fondation Leducq (grant TNE-18CVD04), and Massachusetts General Hospital (Paul and Phyllis Fireman Endowed Chair in Vascular Medicine); has received personal consulting fees from Amgen, Apple, AstraZeneca, Blackstone Life Sciences, Foresite Labs, Genentech, Novartis, and TenSixteen Bio; has received investigator-initiated grants from Apple, AstraZeneca, and Boston Scientific; is a co-founder of TenSixteen Bio; has equity in TenSixteen Bio, geneXwell, and Vertex; and has spousal employment at Vertex, all unrelated to the present work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



Comment in
-
Lipoprotein(a) and Oxidized Phospholipids: Partners in Crime or Individual Perpetrators in Cardiovascular Disease?J Am Coll Cardiol. 2023 May 9;81(18):1793-1796. doi: 10.1016/j.jacc.2023.02.051. J Am Coll Cardiol. 2023. PMID: 37137589 No abstract available.
References
-
- Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics—2021 Update. Circulation. 2021;143:e254–e743. - PubMed
-
- Miksenas H, Januzzi JL, Natarajan P. Lipoprotein(a) and Cardiovascular Diseases. JAMA. 2021;326:352–353. - PubMed
-
- Trégouët D-A, König IR, Erdmann J, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–285. - PubMed

