Rationale and design of a randomised trial of trientine in patients with hypertrophic cardiomyopathy
- PMID: 37137675
- PMCID: PMC10359575
- DOI: 10.1136/heartjnl-2022-322271
Rationale and design of a randomised trial of trientine in patients with hypertrophic cardiomyopathy
Abstract
Aims: Hypertrophic cardiomyopathy (HCM) is characterised by left ventricular hypertrophy (LVH), myocardial fibrosis, enhanced oxidative stress and energy depletion. Unbound/loosely bound tissue copper II ions are powerful catalysts of oxidative stress and inhibitors of antioxidants. Trientine is a highly selective copper II chelator. In preclinical and clinical studies in diabetes, trientine is associated with reduced LVH and fibrosis, and improved mitochondrial function and energy metabolism. Trientine was associated with improvements in cardiac structure and function in an open-label study in patients with HCM.
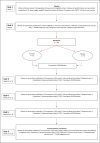
Methods: The Efficacy and Mechanism of Trientine in Patients with Hypertrophic Cardiomyopathy (TEMPEST) trial is a multicentre, double-blind, parallel group, 1:1 randomised, placebo-controlled phase II trial designed to evaluate the efficacy and mechanism of action of trientine in patients with HCM. Patients with a diagnosis of HCM according to the European Society of Cardiology Guidelines and in New York Heart Association classes I-III are randomised to trientine or matching placebo for 52 weeks. Primary outcome is change in left ventricular (LV) mass indexed to body surface area, measured using cardiovascular magnetic resonance. Secondary efficacy objectives will determine whether trientine improves exercise capacity, reduces arrhythmia burden, reduces cardiomyocyte injury, improves LV and atrial function, and reduces LV outflow tract gradient. Mechanistic objectives will determine whether the effects are mediated by cellular or extracellular mass regression and improved myocardial energetics.
Conclusion: TEMPEST will determine the efficacy and mechanism of action of trientine in patients with HCM.
Trial registration numbers: NCT04706429 and ISRCTN57145331.
Keywords: cardiomyopathy, hypertrophic; hypertrophic cardiomyopathy; pharmacology, clinical.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: Relationships with industry are as follows: Univar Solutions B.V. has gifted the investigational medicinal product. Univar Solutions B.V. have had no role in trial design, the preparation or approval of this manuscript, and the decision to submit the manuscript for publication. Univar Solutions B.V. conducted a factual accuracy check of this manuscript, but any decisions to incorporate comments were made solely at the discretion of the authors. BR is advisor for Axcella Therapeutics and patent inventor of a new oxygen sensitive MRI approach USPTO, Serial No. 16/674,104, Nov 5, 2019 Serial No. GB 1818147.9, Nov 7, 2018. CAM has served as an advisor for AstraZeneca, Boehringer Ingelheim and Lilly Alliance, Novartis, PureTech Health and HAYA Therapeutics; and has received research support from Amicus Therapeutics, Guerbet Laboratories Limited and Roche.
Figures
References
-
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/american Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol 2020;76:e159–240. 10.1016/j.jacc.2020.08.045 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
