Paradoxes of Cellular SUMOylation Regulation: A Role of Biomolecular Condensates?
- PMID: 37137717
- PMCID: PMC10441629
- DOI: 10.1124/pharmrev.122.000784
Paradoxes of Cellular SUMOylation Regulation: A Role of Biomolecular Condensates?
Abstract
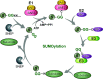
Protein SUMOylation is a major post-translational modification essential for maintaining cellular homeostasis. SUMOylation has long been associated with stress responses as a diverse array of cellular stress signals are known to trigger rapid alternations in global protein SUMOylation. In addition, while there are large families of ubiquitination enzymes, all small ubiquitin-like modifiers (SUMOs) are conjugated by a set of enzymatic machinery comprising one heterodimeric SUMO-activating enzyme, a single SUMO-conjugating enzyme, and a small number of SUMO protein ligases and SUMO-specific proteases. How a few SUMOylation enzymes specifically modify thousands of functional targets in response to diverse cellular stresses remains an enigma. Here we review recent progress toward understanding the mechanisms of SUMO regulation, particularly the potential roles of liquid-liquid phase separation/biomolecular condensates in regulating cellular SUMOylation during cellular stresses. In addition, we discuss the role of protein SUMOylation in pathogenesis and the development of novel therapeutics targeting SUMOylation. SIGNIFICANCE STATEMENT: Protein SUMOylation is one of the most prevalent post-translational modifications and plays a vital role in maintaining cellular homeostasis in response to stresses. Protein SUMOylation has been implicated in human pathogenesis, such as cancer, cardiovascular diseases, neurodegeneration, and infection. After more than a quarter century of extensive research, intriguing enigmas remain regarding the mechanism of cellular SUMOylation regulation and the therapeutic potential of targeting SUMOylation.
Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
SUMO: From Bench to Bedside.Physiol Rev. 2020 Oct 1;100(4):1599-1619. doi: 10.1152/physrev.00025.2019. Physiol Rev. 2020. PMID: 32666886 Free PMC article. Review.
-
Molecular mechanisms in SUMO conjugation.Biochem Soc Trans. 2020 Feb 28;48(1):123-135. doi: 10.1042/BST20190357. Biochem Soc Trans. 2020. PMID: 31872228 Review.
-
Identification of biochemically distinct properties of the small ubiquitin-related modifier (SUMO) conjugation pathway in Plasmodium falciparum.J Biol Chem. 2013 Sep 27;288(39):27724-36. doi: 10.1074/jbc.M113.498410. Epub 2013 Aug 13. J Biol Chem. 2013. PMID: 23943616 Free PMC article.
-
Signalling mechanisms and cellular functions of SUMO.Nat Rev Mol Cell Biol. 2022 Nov;23(11):715-731. doi: 10.1038/s41580-022-00500-y. Epub 2022 Jun 24. Nat Rev Mol Cell Biol. 2022. PMID: 35750927 Review.
-
The SUMO Pathway.Methods Mol Biol. 2025;2957:1-15. doi: 10.1007/978-1-0716-4710-3_1. Methods Mol Biol. 2025. PMID: 40875111 Review.
Cited by
-
Biomolecular phase separation in tumorigenesis: from aberrant condensates to therapeutic vulnerabilities.Mol Cancer. 2025 Aug 23;24(1):220. doi: 10.1186/s12943-025-02428-1. Mol Cancer. 2025. PMID: 40849651 Free PMC article. Review.
-
Ubiquitin-modifying enzymes in thyroid cancer:Mechanisms and functions.Heliyon. 2024 Jul 2;10(13):e34032. doi: 10.1016/j.heliyon.2024.e34032. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39091932 Free PMC article. Review.
-
Protein SUMOylation promotes cAMP-independent EPAC1 activation.Cell Mol Life Sci. 2024 Jul 4;81(1):283. doi: 10.1007/s00018-024-05315-y. Cell Mol Life Sci. 2024. PMID: 38963422 Free PMC article.
-
Single-Cell Spatial-Temporal Analysis of ZNF451 in Mediating Drug Resistance and CD8+ T Cell Dysfunction.Research (Wash D C). 2024 Nov 12;7:0530. doi: 10.34133/research.0530. eCollection 2024. Research (Wash D C). 2024. PMID: 39534688 Free PMC article.
-
Heat Shock Strengthens the Protective Potential of MSCs in Liver Injury by Promoting EV Release Through Upregulated Autophagosome Formation.J Extracell Vesicles. 2025 May;14(5):e70084. doi: 10.1002/jev2.70084. J Extracell Vesicles. 2025. PMID: 40326673 Free PMC article.
References
-
- Alberti S, Dormann D (2019) Liquid-liquid phase separation in disease. Annu Rev Genet 53:171–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

