Associations of CSF PDGFRβ With Aging, Blood-Brain Barrier Damage, Neuroinflammation, and Alzheimer Disease Pathologic Changes
- PMID: 37137722
- PMCID: PMC10351311
- DOI: 10.1212/WNL.0000000000207358
Associations of CSF PDGFRβ With Aging, Blood-Brain Barrier Damage, Neuroinflammation, and Alzheimer Disease Pathologic Changes
Abstract
Background and objectives: Injured pericytes in the neurovascular unit release platelet-derived growth factor β (PDGFRβ) into the CSF. However, it is not clear how pericyte injury contributes to Alzheimer disease (AD)-related changes and blood-brain barrier (BBB) damage. We aimed to test whether CSF PDGFRβ was associated with different AD-associated and age-associated pathologic changes leading to dementia.
Methods: PDGFRβ was measured in the CSF of 771 participants with cognitively unimpaired (CU, n = 408), mild cognitive impairment (MCI, n = 175), and dementia (n = 188) from the Swedish BioFINDER-2 cohort. We then checked association with β-amyloid (Aβ)-PET and tau-PET standardized uptake value ratio, APOE ε4 genotype and MRI measurements of cortical thickness, white matter lesions (WMLs), and cerebral blood flow. We also analyzed the role of CSF PDGFRβ in the relationship between aging, BBB dysfunction (measured by CSF/plasma albumin ratio, QAlb), and neuroinflammation (i.e., CSF levels of YKL-40 and glial fibrillary acidic protein [GFAP], preferentially expressed in reactive astrocytes).
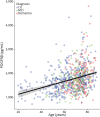
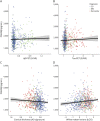
Results: The cohort had a mean age of 67 years (CU = 62.8, MCI = 69.9, dementia = 70.4), and 50.1% were male (CU = 46.6%, MCI = 53.7%, dementia = 54.3%). Higher CSF PDGFRβ concentrations were related to higher age (b = 19.1, β = 0.5, 95% CI 16-22.2, p < 0.001), increased CSF neuroinflammatory markers of glial activation YKL-40 (b = 3.4, β = 0.5, 95% CI 2.8-3.9, p < 0.001), GFAP (b = 27.4, β = 0.4, 95% CI 20.9-33.9, p < 0.001), and worse BBB integrity measured by QAlb (b = 37.4, β = 0.2, 95% CI 24.9-49.9, p < 0.001). Age was also associated with worse BBB integrity, and this was partly mediated by PDGFRβ and neuroinflammatory markers (16%-33% of total effect). However, PDGFRβ showed no associations with APOE ε4 genotype, PET imaging of Aβ and tau pathology, or MRI measures of brain atrophy and WMLs (p > 0.05).
Discussion: In summary, pericyte damage, reflected by CSF PDGFRβ, may be involved in age-related BBB disruption together with neuroinflammation, but is not related to Alzheimer-related pathologic changes.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
C. Cicognola, N. Mattsson-Carlgren, and D. van Westen report no disclosures relevant to the manuscript. H. Zetterberg has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K. Blennow has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this article. S. Palmqvist has served on scientific advisory boards and/or given lectures in symposia sponsored by Bioartic, Biogen, Eli Lilly, Geras Solutions, and Roche. K. Ahmadi, O. Strandberg, E. Stomrud, and S. Janelidze report no disclosures relevant to the manuscript. O. Hansson has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens. Go to
Figures


Similar articles
-
Dissection of blood-brain barrier dysfunction through CSF PDGFRβ and amyloid, tau, neuroinflammation, and synaptic CSF biomarkers in neurodegenerative disorders.EBioMedicine. 2025 May;115:105694. doi: 10.1016/j.ebiom.2025.105694. Epub 2025 Apr 15. EBioMedicine. 2025. PMID: 40239464 Free PMC article.
-
Assessing blood-brain barrier dysfunction and its association with Alzheimer's pathology, cognitive impairment and neuroinflammation.Alzheimers Res Ther. 2024 Jul 31;16(1):172. doi: 10.1186/s13195-024-01529-1. Alzheimers Res Ther. 2024. PMID: 39085945 Free PMC article.
-
Evidence of Pericyte Damage in a Cognitively Normal Cohort: Association With CSF and PET Biomarkers of Alzheimer Disease.Alzheimer Dis Assoc Disord. 2024 Apr-Jun 01;38(2):107-111. doi: 10.1097/WAD.0000000000000623. Epub 2024 May 16. Alzheimer Dis Assoc Disord. 2024. PMID: 38752577 Free PMC article.
-
Serum proteomics reveals early biomarkers of Alzheimer's disease: The dual role of APOE-ε4.Biosci Trends. 2025 Mar 6;19(1):1-9. doi: 10.5582/bst.2024.01365. Epub 2025 Jan 23. Biosci Trends. 2025. PMID: 39842814 Review.
-
Blood Astrocyte Biomarkers in Alzheimer Disease: A Systematic Review and Meta-Analysis.Neurology. 2024 Aug 13;103(3):e209537. doi: 10.1212/WNL.0000000000209537. Epub 2024 Jul 10. Neurology. 2024. PMID: 38986050 Free PMC article.
Cited by
-
Blood-brain barrier disruption: a culprit of cognitive decline?Fluids Barriers CNS. 2024 Aug 7;21(1):63. doi: 10.1186/s12987-024-00563-3. Fluids Barriers CNS. 2024. PMID: 39113115 Free PMC article. Review.
-
Cerebrospinal Fluid Biomarkers in Opioid Dependence: Evidence of Neuroimmune Activation and Ion Composition Changes, Without Alteration in Orexin-A.Addict Biol. 2025 Jun;30(6):e70053. doi: 10.1111/adb.70053. Addict Biol. 2025. PMID: 40454659 Free PMC article.
-
Ion concentrations in CSF and serum are differentially and precisely regulated.Brain Commun. 2025 May 24;7(3):fcaf201. doi: 10.1093/braincomms/fcaf201. eCollection 2025. Brain Commun. 2025. PMID: 40462862 Free PMC article.
-
Ladostigil Reduces the Adenoside Triphosphate/Lipopolysaccharide-Induced Secretion of Pro-Inflammatory Cytokines from Microglia and Modulate-Immune Regulators, TNFAIP3, and EGR1.Biomolecules. 2024 Jan 16;14(1):112. doi: 10.3390/biom14010112. Biomolecules. 2024. PMID: 38254713 Free PMC article.
-
Fluid biomarkers in cerebral amyloid angiopathy.Front Neurosci. 2024 Jan 26;18:1347320. doi: 10.3389/fnins.2024.1347320. eCollection 2024. Front Neurosci. 2024. PMID: 38344467 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
