Pregnancy Outcomes in Patients Exposed to OnabotulinumtoxinA Treatment: A Cumulative 29-Year Safety Update
- PMID: 37137724
- PMCID: PMC10351549
- DOI: 10.1212/WNL.0000000000207375
Pregnancy Outcomes in Patients Exposed to OnabotulinumtoxinA Treatment: A Cumulative 29-Year Safety Update
Erratum in
-
Pregnancy Outcomes in Patients Exposed to OnabotulinumtoxinA Treatment: A Cumulative 29-Year Safety Update.Neurology. 2025 Jul 22;105(2):e213772. doi: 10.1212/WNL.0000000000213772. Epub 2025 Jun 26. Neurology. 2025. PMID: 40570277 Free PMC article. No abstract available.
Abstract
Background and objectives: A previous publication of pregnancy outcomes in onabotulinumtoxinA-exposed mothers demonstrated that the prevalence of major fetal defects (0.9%, 1/110) was comparable with background rates in the general population. There is continued interest to better understand the safety of onabotulinumtoxinA during pregnancy. This analysis evaluated pregnancy outcomes after onabotulinumtoxinA exposure to provide a cumulative 29-year update.
Methods: The Allergan Global Safety Database was searched from January 1, 1990, to December 31, 2018. Data from women (younger than 65 years or unknown) during pregnancy or ≤3 months before conception treated with onabotulinumtoxinA were assessed to estimate birth defect prevalence rates of live births only from prospective pregnancies.
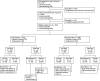
Results: Of 913 pregnancies, 397 (43.5%) were eligible with known outcomes. Maternal age was known in 215 pregnancies: 45.6% were 35 years or older. Indication was known in 340 pregnancies: most frequent were aesthetic (35.3%) and migraine/headache (30.3%). The timing of exposure was known in 318 pregnancies: 94.6% were before conception or during the first trimester. OnabotulinumtoxinA dose information was known in 242 pregnancies; most (83.5%) were exposed to <200 U. Of 195 prospective pregnancies with 197 fetuses, there were 152 (77.2%) live births and 45 (22.8%) fetal losses (32 spontaneous, 13 elective). Of 152 live births, 148 (97.4%) had normal outcomes and 4 had abnormal outcomes. Among the 4 abnormal outcomes, there were 1 major birth defect, 2 minor fetal defects, and 1 birth complication. The prevalence rate for overall fetal defects was 2.6% (4/152, 95% CI 1.0%-6.6%) and 0.7% (1/152, 95% CI 0.1%-3.6%) for major fetal defects (3%-6% in the general population). Among cases of live births and known determinable exposure times, there was 1 birth defect with preconception exposure and 2 with first-trimester exposure.
Discussion: Although subject to reporting bias due to the nature of the postmarketing database review, this 29-year retrospective analysis of safety data in pregnant women exposed to onabotulinumtoxinA demonstrates that the prevalence rate of major fetal defects among live births is consistent with the rates reported in the general population. Although there are limited data available for second-trimester and third-trimester exposure, this updated and expanded safety analysis provides important real-world evidence to health care providers and their patients.
Classification of evidence: This analysis provides Class III data that demonstrate that the prevalence rate of major fetal defects among live births subsequent to in utero onabotulinumtoxinA exposure is comparable with the reported background rates.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
Neither honoraria nor any other form of compensation was provided for authorship. Financial arrangements, within the past year, of the authors with companies whose products may be related to the present manuscript are listed, as declared by the authors. M.F. Brin: employee of AbbVie and receives salary and stock/stock options as compensation. R.S. Kirby: received grant funding from the Centers for Disease Control and Prevention, Florida Department of Health, and Maternal and Child Health Bureau/Health Resources and Services Administration; employee of University of South Florida. A. Slavotinek: none reported as relevant to this publication. A.M. Adams: employee of AbbVie and receives salary and stock/stock options as compensation. L. Parker: employee of AbbVie and receives salary as compensation. A. Ukah: employee of AbbVie and receives salary and stock/stock options as compensation. L. Radulian: employee of AbbVie and receives salary and stock/stock options as compensation and is a PhD student at the University of Medicine and Pharmacy. M.R.P. Elmore: employee of AbbVie and receives salary and stock/stock options as compensation. L. Yedigarova: former employee of AbbVie. I. Yushmanova: employee of AbbVie and receives salary and stock/stock options as compensation. M.F. Brin and L. Parker had full access to all the data in the analysis and take responsibility for the integrity of the data and the accuracy of the data analysis. Go to
Figures
Comment in
-
Periconceptional Use of OnabotulinumtoxinA: Need for Comparative Safety Studies.Neurology. 2023 Jul 11;101(2):53-54. doi: 10.1212/WNL.0000000000207469. Epub 2023 May 3. Neurology. 2023. PMID: 37137723 No abstract available.
References
-
- Brin MF. Development of future indications for BOTOX. Toxicon. 2009;54(5):668-674. - PubMed
-
- Botox® (onabotulinumtoxinA) [prescribing information]. AbbVie Inc.; 2022.
-
- Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818-1826. - PMC - PubMed
-
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30(9):1065-1072. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials