Structural atlas of a human gut crassvirus
- PMID: 37138077
- PMCID: PMC10172136
- DOI: 10.1038/s41586-023-06019-2
Structural atlas of a human gut crassvirus
Abstract
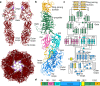
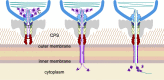
CrAssphage and related viruses of the order Crassvirales (hereafter referred to as crassviruses) were originally discovered by cross-assembly of metagenomic sequences. They are the most abundant viruses in the human gut, are found in the majority of individual gut viromes, and account for up to 95% of the viral sequences in some individuals1-4. Crassviruses are likely to have major roles in shaping the composition and functionality of the human microbiome, but the structures and roles of most of the virally encoded proteins are unknown, with only generic predictions resulting from bioinformatic analyses4,5. Here we present a cryo-electron microscopy reconstruction of Bacteroides intestinalis virus ΦcrAss0016, providing the structural basis for the functional assignment of most of its virion proteins. The muzzle protein forms an assembly about 1 MDa in size at the end of the tail and exhibits a previously unknown fold that we designate the 'crass fold', that is likely to serve as a gatekeeper that controls the ejection of cargos. In addition to packing the approximately 103 kb of virus DNA, the ΦcrAss001 virion has extensive storage space for virally encoded cargo proteins in the capsid and, unusually, within the tail. One of the cargo proteins is present in both the capsid and the tail, suggesting a general mechanism for protein ejection, which involves partial unfolding of proteins during their extrusion through the tail. These findings provide a structural basis for understanding the mechanisms of assembly and infection of these highly abundant crassviruses.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Similar articles
-
Structural basis for scaffolding-mediated assembly and maturation of a dsDNA virus.Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1355-60. doi: 10.1073/pnas.1015739108. Epub 2011 Jan 10. Proc Natl Acad Sci U S A. 2011. PMID: 21220301 Free PMC article.
-
Cryo-EM structure of a Marseilleviridae virus particle reveals a large internal microassembly.Virology. 2018 Mar;516:239-245. doi: 10.1016/j.virol.2018.01.021. Virology. 2018. PMID: 29407382
-
Structural Insights into the Assembly of the African Swine Fever Virus Inner Capsid.J Virol. 2023 Jun 29;97(6):e0026823. doi: 10.1128/jvi.00268-23. Epub 2023 May 16. J Virol. 2023. PMID: 37191520 Free PMC article.
-
The Ins and Outs of Herpesviral Capsids: Divergent Structures and Assembly Mechanisms across the Three Subfamilies.Viruses. 2021 Sep 23;13(10):1913. doi: 10.3390/v13101913. Viruses. 2021. PMID: 34696343 Free PMC article. Review.
-
Seeing and touching adenovirus: complementary approaches for understanding assembly and disassembly of a complex virion.Curr Opin Virol. 2022 Feb;52:112-122. doi: 10.1016/j.coviro.2021.11.006. Epub 2021 Dec 11. Curr Opin Virol. 2022. PMID: 34906758 Review.
Cited by
-
VTX: real-time high-performance molecular structure and dynamics visualization software.Bioinformatics. 2025 Jun 2;41(6):btaf295. doi: 10.1093/bioinformatics/btaf295. Bioinformatics. 2025. PMID: 40353852 Free PMC article.
-
Conformational changes in and translocation of small proteins: insights into the ejection mechanism of podophages.J Virol. 2025 Jan 31;99(1):e0124924. doi: 10.1128/jvi.01249-24. Epub 2024 Dec 20. J Virol. 2025. PMID: 39704524 Free PMC article.
-
Host interactions of novel Crassvirales species belonging to multiple families infecting bacterial host, Bacteroides cellulosilyticus WH2.Microb Genom. 2023 Sep;9(9):001100. doi: 10.1099/mgen.0.001100. Microb Genom. 2023. PMID: 37665209 Free PMC article.
-
Decoding huge phage diversity: a taxonomic classification of Lak megaphages.J Gen Virol. 2024 May;105(5):001997. doi: 10.1099/jgv.0.001997. J Gen Virol. 2024. PMID: 38814706 Free PMC article.
-
Megataxonomy and global ecology of the virosphere.ISME J. 2024 Jan 8;18(1):wrad042. doi: 10.1093/ismejo/wrad042. ISME J. 2024. PMID: 38365236 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

