Glioblastoma remodelling of human neural circuits decreases survival
- PMID: 37138086
- PMCID: PMC10191851
- DOI: 10.1038/s41586-023-06036-1
Glioblastoma remodelling of human neural circuits decreases survival
Abstract
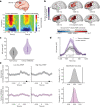
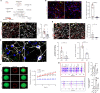
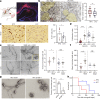
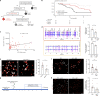
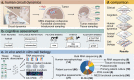
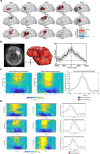
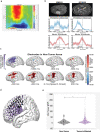
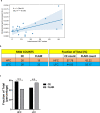
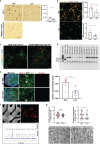
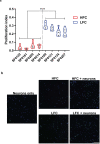
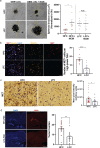
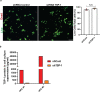
Gliomas synaptically integrate into neural circuits1,2. Previous research has demonstrated bidirectional interactions between neurons and glioma cells, with neuronal activity driving glioma growth1-4 and gliomas increasing neuronal excitability2,5-8. Here we sought to determine how glioma-induced neuronal changes influence neural circuits underlying cognition and whether these interactions influence patient survival. Using intracranial brain recordings during lexical retrieval language tasks in awake humans together with site-specific tumour tissue biopsies and cell biology experiments, we find that gliomas remodel functional neural circuitry such that task-relevant neural responses activate tumour-infiltrated cortex well beyond the cortical regions that are normally recruited in the healthy brain. Site-directed biopsies from regions within the tumour that exhibit high functional connectivity between the tumour and the rest of the brain are enriched for a glioblastoma subpopulation that exhibits a distinct synaptogenic and neuronotrophic phenotype. Tumour cells from functionally connected regions secrete the synaptogenic factor thrombospondin-1, which contributes to the differential neuron-glioma interactions observed in functionally connected tumour regions compared with tumour regions with less functional connectivity. Pharmacological inhibition of thrombospondin-1 using the FDA-approved drug gabapentin decreases glioblastoma proliferation. The degree of functional connectivity between glioblastoma and the normal brain negatively affects both patient survival and performance in language tasks. These data demonstrate that high-grade gliomas functionally remodel neural circuits in the human brain, which both promotes tumour progression and impairs cognition.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
How thought itself can drive tumour growth.Nature. 2023 May;617(7961):469-471. doi: 10.1038/d41586-023-01387-1. Nature. 2023. PMID: 37138055 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- U19 CA264504/CA/NCI NIH HHS/United States
- U19 CA264338/CA/NCI NIH HHS/United States
- R01 CA258384/CA/NCI NIH HHS/United States
- R01 NS092597/NS/NINDS NIH HHS/United States
- R21 CA264339/CA/NCI NIH HHS/United States
- K99 CA252001/CA/NCI NIH HHS/United States
- P50 CA097257/CA/NCI NIH HHS/United States
- R01 NS100440/NS/NINDS NIH HHS/United States
- K08 CA212279/CA/NCI NIH HHS/United States
- P50 CA165962/CA/NCI NIH HHS/United States
- K08 NS110919/NS/NINDS NIH HHS/United States
- R01 CA251221/CA/NCI NIH HHS/United States
- DP1 NS111132/NS/NINDS NIH HHS/United States
- R01 CA263500/CA/NCI NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- R00 DC013828/DC/NIDCD NIH HHS/United States
- F30 CA246808/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

