Twist exome capture allows for lower average sequence coverage in clinical exome sequencing
- PMID: 37138343
- PMCID: PMC10155375
- DOI: 10.1186/s40246-023-00485-5
Twist exome capture allows for lower average sequence coverage in clinical exome sequencing
Abstract
Background: Exome and genome sequencing are the predominant techniques in the diagnosis and research of genetic disorders. Sufficient, uniform and reproducible/consistent sequence coverage is a main determinant for the sensitivity to detect single-nucleotide (SNVs) and copy number variants (CNVs). Here we compared the ability to obtain comprehensive exome coverage for recent exome capture kits and genome sequencing techniques.
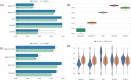
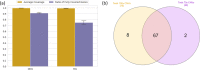
Results: We compared three different widely used enrichment kits (Agilent SureSelect Human All Exon V5, Agilent SureSelect Human All Exon V7 and Twist Bioscience) as well as short-read and long-read WGS. We show that the Twist exome capture significantly improves complete coverage and coverage uniformity across coding regions compared to other exome capture kits. Twist performance is comparable to that of both short- and long-read whole genome sequencing. Additionally, we show that even at a reduced average coverage of 70× there is only minimal loss in sensitivity for SNV and CNV detection.
Conclusion: We conclude that exome sequencing with Twist represents a significant improvement and could be performed at lower sequence coverage compared to other exome capture techniques.
Keywords: Exome sequencing; Genome sequencing; Uniformity of coverage.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

