Molecular and pathological investigation of avian reovirus (ARV) in Egypt with the assessment of the genetic variability of field strains compared to vaccine strains
- PMID: 37138631
- PMCID: PMC10150020
- DOI: 10.3389/fmicb.2023.1156251
Molecular and pathological investigation of avian reovirus (ARV) in Egypt with the assessment of the genetic variability of field strains compared to vaccine strains
Abstract
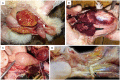
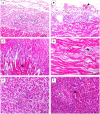
Avian orthoreovirus (ARV) is among the important viruses that cause drastic economic losses in the Egyptian poultry industry. Despite regular vaccination of breeder birds, a high prevalence of ARV infection in broilers has been noted in recent years. However, no reports have revealed the genetic and antigenic characteristics of Egyptian field ARV and vaccines used against it. Thus, this study was conducted to detect the molecular nature of emerging ARV strains in broiler chickens suffering from arthritis and tenosynovitis in comparison to vaccine strains. Synovial fluid samples (n = 400) were collected from 40 commercial broiler flocks in the Gharbia governorate, Egypt, and then pooled to obtain 40 samples, which were then used to screen ARV using reverse transcriptase polymerase chain reaction (RT-PCR) with the partial amplification of ARV sigma C gene. The obtained RT-PCR products were then sequenced, and their nucleotide and deduced amino acid sequences were analyzed together with other ARV field and vaccine strains from GenBank. RT-PCR successfully amplified the predicted 940 bp PCR products from all tested samples. The phylogenetic tree revealed that the analyzed ARV strains were clustered into six genotypic clusters and six protein clusters, with high antigenic diversity between the genotypic clusters. Surprisingly, our isolates were genetically different from vaccine strains, which aligned in genotypic cluster I/protein cluster I, while our strains were aligned in genotypic cluster V/protein cluster V. More importantly, our strains were highly divergent from vaccine strains used in Egypt, with 55.09-56.23% diversity. Sequence analysis using BioEdit software revealed high genetic and protein diversity between our isolates and vaccine strains (397/797 nucleotide substitutions and 148-149/265 amino acid substitutions). This high genetic diversity explains the vaccination failure and recurrent circulation of ARV in Egypt. The present data highlight the need to formulate a new effective vaccine from locally isolated ARV strains after a thorough screening of the molecular nature of circulating ARV in Egypt.
Keywords: ARV; avian orthoreovirus; histopathology; phylogenetic analysis; sigma C; vaccine.
Copyright © 2023 Mosad, Elmahallawy, Alghamdi, El-Khayat, El-Khadragy, Ali and Abdo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Avian Orthoreoviruses: A Systematic Review of Their Distribution, Dissemination Patterns, and Genotypic Clustering.Viruses. 2024 Jun 29;16(7):1056. doi: 10.3390/v16071056. Viruses. 2024. PMID: 39066218 Free PMC article.
-
First report of seroprevalence and genetic characterization of avian orthoreovirus in Egypt.Trop Anim Health Prod. 2020 May;52(3):1049-1054. doi: 10.1007/s11250-019-02100-z. Epub 2019 Nov 8. Trop Anim Health Prod. 2020. PMID: 31705354
-
Genetic characterization of newly emerging avian reovirus variants in chickens with viral arthritis/tenosynovitis in Israel.Virology. 2024 Jan;589:109908. doi: 10.1016/j.virol.2023.109908. Epub 2023 Oct 10. Virology. 2024. PMID: 37952464
-
Pathogenicity and genomic characterization of a novel avian orthoreovius variant isolated from a vaccinated broiler flock in China.Avian Pathol. 2019 Aug;48(4):334-342. doi: 10.1080/03079457.2019.1600656. Epub 2019 Apr 18. Avian Pathol. 2019. PMID: 30915860
-
Avian Reovirus in Israel, Variants and Vaccines-A Review.Avian Dis. 2022 Dec;66(4):447-451. doi: 10.1637/aviandiseases-D-22-99996. Avian Dis. 2022. PMID: 36715478 Review.
Cited by
-
Avian Orthoreoviruses: A Systematic Review of Their Distribution, Dissemination Patterns, and Genotypic Clustering.Viruses. 2024 Jun 29;16(7):1056. doi: 10.3390/v16071056. Viruses. 2024. PMID: 39066218 Free PMC article.
-
Avian Reovirus: From Molecular Biology to Pathogenesis and Control.Viruses. 2024 Dec 23;16(12):1966. doi: 10.3390/v16121966. Viruses. 2024. PMID: 39772272 Free PMC article. Review.
References
-
- Abd El-Samie L. (2014). Some causes of chicken's growth retardation in sharkia, Egypt. Assiut. Vet. Med. J. 61, 32–37. 10.21608/avmj.2014.169747 - DOI
-
- Attoui H. (2011). “Family reoviridae in virus taxonomy: classification and nomenclature of viruses,” in Ninth Report of the International Committee on Taxonomy of Viruses 541–54. - PubMed
LinkOut - more resources
Full Text Sources

