Understanding CD4+ T cells in autoimmune bullous diseases
- PMID: 37138879
- PMCID: PMC10149917
- DOI: 10.3389/fimmu.2023.1161927
Understanding CD4+ T cells in autoimmune bullous diseases
Abstract
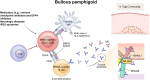
Autoimmune bullous diseases (AIBDs) are a group of life-threatening blistering diseases caused by autoantibodies that target proteins in the skin and mucosa. Autoantibodies are the most important mediator in the pathogenesis of AIBDs, and various immune mechanisms contribute to the production of these pathogenic autoantibodies. Recently, significant progress has been made in understanding how CD4+ T cells drive autoantibody production in these diseases. Here, we review the critical role of CD4+ T cells in the production of pathogenic autoantibodies for the initiation and perpetuation of humoral response in AIBDs. To gain an in-depth understanding of CD4+ T-cell pathogenicity, antigen specificity, and mechanisms of immune tolerance, this review covers comprehensive mouse and human studies of pemphigus and bullous pemphigoid. Further exploration of pathogenic CD4+ T cells will potentially provide immune targets for improved treatment of AIBDs.
Keywords: CD4 T cells; autoimmune bullous disease; bullous pemphigoid; pathogenicity; pemphigus.
Copyright © 2023 Lee, Kim and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Regulatory T-cell deficiency leads to pathogenic bullous pemphigoid antigen 230 autoantibody and autoimmune bullous disease.J Allergy Clin Immunol. 2018 Dec;142(6):1831-1842.e7. doi: 10.1016/j.jaci.2018.04.006. Epub 2018 Apr 26. J Allergy Clin Immunol. 2018. PMID: 29704595
-
Classification and Antigen Molecules of Autoimmune Bullous Diseases.Biomolecules. 2023 Apr 20;13(4):703. doi: 10.3390/biom13040703. Biomolecules. 2023. PMID: 37189450 Free PMC article. Review.
-
Autoimmune blistering diseases: promising agents in clinical trials.Expert Opin Investig Drugs. 2023 Jul-Dec;32(7):615-623. doi: 10.1080/13543784.2023.2242778. Epub 2023 Aug 7. Expert Opin Investig Drugs. 2023. PMID: 37526503 Review.
-
Evidence for pathogenicity of autoreactive T cells in autoimmune bullous diseases shown by animal disease models.Exp Dermatol. 2012 Dec;21(12):901-5. doi: 10.1111/exd.12011. Epub 2012 Sep 28. Exp Dermatol. 2012. PMID: 23016514
-
Current Clinical Trials in Pemphigus and Pemphigoid.Front Immunol. 2019 May 3;10:978. doi: 10.3389/fimmu.2019.00978. eCollection 2019. Front Immunol. 2019. PMID: 31130959 Free PMC article. Review.
Cited by
-
A Case of Bullous Pemphigoid with Significant Infiltration of CD4-Positive T Cells during Treatment with Pembrolizumab, Accompanied by Pembrolizumab-Induced Multi-Organ Dysfunction.Diagnostics (Basel). 2024 Sep 4;14(17):1958. doi: 10.3390/diagnostics14171958. Diagnostics (Basel). 2024. PMID: 39272742 Free PMC article.
-
Microenvironmental network of clonal CXCL13+CD4+ T cells and Tregs in pemphigus chronic blisters.J Clin Invest. 2023 Dec 1;133(23):e166357. doi: 10.1172/JCI166357. J Clin Invest. 2023. PMID: 37815865 Free PMC article. Clinical Trial.
-
JAK-STAT pathway, type I/II cytokines, and new potential therapeutic strategy for autoimmune bullous diseases: update on pemphigus vulgaris and bullous pemphigoid.Front Immunol. 2025 Apr 8;16:1563286. doi: 10.3389/fimmu.2025.1563286. eCollection 2025. Front Immunol. 2025. PMID: 40264772 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

