Short-term effects of intravenous batroxobin in treatment of sudden sensorineural hearing loss: a propensity score-matched study
- PMID: 37139065
- PMCID: PMC10150045
- DOI: 10.3389/fneur.2023.1102297
Short-term effects of intravenous batroxobin in treatment of sudden sensorineural hearing loss: a propensity score-matched study
Abstract
Background: Sudden sensorineural hearing loss (SSNHL) can cause great panic in patients. Whether it is advantageous to add intravenous batroxobin in the treatment of SSNHL remains to be determined. This study aimed to compare the short-term efficacy of therapy combined with intravenous batroxobin and that without intravenous batroxobin in SSNHL patients.
Methods: This retrospective study harvested the data of SSNHL patients hospitalized in our department from January 2008 to April 2021. The hearing levels on the admitted day (before treatment) and the discharge day were considered pre-treatment hearing and post-treatment hearing, respectively. The hearing gain was the difference value of pre-treatment hearing and post-treatment hearing. We used Siegel's criteria and the Chinese Medical Association of Otolaryngology (CMAO) criteria to evaluate hearing recovery. The complete recovery rate, overall effective rate, and hearing gain at each frequency were considered outcomes. Propensity score matching (PSM) was conducted to balance the baseline characteristics between the batroxobin group and the non-batroxobin group. Sensitivity analysis was carried out in flat-type and total-deafness SSNHL patients.
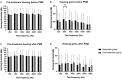
Results: During the study period, 657 patients with SSNHL were admitted to our department. Among them, a total of 274 patients met the enrolled criteria of our study. After PSM, 162 patients (81 in each group) were included in the analysis. Once the hospitalized treatment was completed, the patients would be discharged the next day. Logistic regression analysis of the propensity score-matched cohort indicated that both the complete recovery rates [Siegel's criteria, OR: 0.734, 95% CI: 0.368-1.466, p = 0.381; CMAO criteria, OR: 0.879, 95% CI: 0.435-1.777, p = 0.720] and the overall effective rates [Siegel's criteria and CMAO criteria, OR: 0.741, 95% CI: 0.399-1.378, p = 0.344] were not significantly different between the two treatment groups. Sensitivity analysis has shown similar results. For flat-type and total-deafness SSNHL patients, no significant difference was found in post-treatment hearing gain at each frequency between the two groups after PSM.
Conclusion: There was no significant difference in short-term hearing outcomes between treatment with batroxobin and treatment without batroxobin in SSNHL patients by Siegel's and CMAO criteria after PSM. Future studies for better therapy regimens of SSNHL are still needed.
Keywords: intravenous batroxobin; propensity score matching; short-term effects; sudden sensorineural hearing loss; treatment.
Copyright © 2023 Jiang, Huang, Mei, He, Cai, Jiang, Wu, Wang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Michel O, Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie. Die aktuell gefasste Leitlinie “Hörsturz” (Akuter idiopathischer sensorineuraler Hörverlust) [The revised version of the german guidelines “sudden idiopathic sensorineural hearing loss”]. Laryngorhinootologie. (2011) 90:290–3. German. 10.1055/s-0031-1273721 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

