Comparative analysis of structural modifications induced by monocular retinal inactivation and monocular deprivation in the developing cat lateral geniculate nucleus
- PMID: 37139534
- PMCID: PMC10330129
- DOI: 10.1002/cne.25493
Comparative analysis of structural modifications induced by monocular retinal inactivation and monocular deprivation in the developing cat lateral geniculate nucleus
Abstract
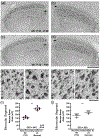
During a critical period of postnatal life, monocular deprivation (MD) by eyelid closure reduces the size of neurons in layers of the dorsal lateral geniculate nucleus (dLGN) connected to the deprived eye and shifts cortical ocular dominance in favor of the non-deprived eye. Temporary inactivation of the non-deprived eye can promote superior recovery from the effects of long-term MD compared to conventional occlusion therapy. In the current study, we assessed the modification of neuron size in the dLGN as a means of measuring the impact of a brief period of monocular inactivation (MI) imposed at different postnatal ages. The biggest impact of MI was observed when it occurred at the peak of the critical period. Unlike the effect of MD, structural plasticity following MI was observed in both the binocular and monocular segments of the dLGN. With increasing age, the capacity for inactivation to alter postsynaptic cell size diminished but was still significant beyond the critical period. In comparison to MD, inactivation produced effects that were about double in magnitude and exhibited efficacy at older ages. Notwithstanding the large neural alterations precipitated by MI, its effects were remediated with a short period of binocular experience, and vision through the previously inactivated eye fully recovered. These results demonstrate that MI is a potent means of modifying the visual pathway and does so at ages when occlusion is ineffective. The efficacy and longevity of inactivation to elicit plasticity highlight its potential to ameliorate disorders of the visual system such as amblyopia.
Keywords: critical period; dorsal lateral geniculate nucleus; monocular deprivation; monocular inactivation; neural plasticity; tetrodotoxin; visual cortex.
© 2023 The Authors. The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures




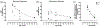


References
-
- Antonini A, Stryker MP (1993). Rapid remodeling of axonal arbors in the visual cortex. Science, 260, 1819–1821. - PubMed
-
- Bach M, Meign T (1999) Do’s and don’ts in Fourier analysis of steady-state potentials. Documenta Ophthalmologica. Advances in Ophthalmology, 99, 69–82. - PubMed
-
- Birch EE, & Stager DR (1988). Prevalence of good visual actuity following surgery for congenital unilateral cataract. Archives of Ophthalmology, 106, 40–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

