Concepts and methods for transcriptome-wide prediction of chemical messenger RNA modifications with machine learning
- PMID: 37139545
- PMCID: PMC10199766
- DOI: 10.1093/bib/bbad163
Concepts and methods for transcriptome-wide prediction of chemical messenger RNA modifications with machine learning
Abstract
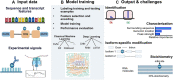
The expanding field of epitranscriptomics might rival the epigenome in the diversity of biological processes impacted. In recent years, the development of new high-throughput experimental and computational techniques has been a key driving force in discovering the properties of RNA modifications. Machine learning applications, such as for classification, clustering or de novo identification, have been critical in these advances. Nonetheless, various challenges remain before the full potential of machine learning for epitranscriptomics can be leveraged. In this review, we provide a comprehensive survey of machine learning methods to detect RNA modifications using diverse input data sources. We describe strategies to train and test machine learning methods and to encode and interpret features that are relevant for epitranscriptomics. Finally, we identify some of the current challenges and open questions about RNA modification analysis, including the ambiguity in predicting RNA modifications in transcript isoforms or in single nucleotides, or the lack of complete ground truth sets to test RNA modifications. We believe this review will inspire and benefit the rapidly developing field of epitranscriptomics in addressing the current limitations through the effective use of machine learning.
Keywords: RNA modifications; deep learning; direct RNA sequencing; epitranscriptomics; machine learning; miCLIP.
© The Author(s) 2023. Published by Oxford University Press.
Figures