First aid with color atlas for the use of intestinal ultrasound for inflammatory bowel disease in daily clinical practice
- PMID: 37139590
- PMCID: PMC10169516
- DOI: 10.5217/ir.2023.00003
First aid with color atlas for the use of intestinal ultrasound for inflammatory bowel disease in daily clinical practice
Abstract
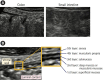
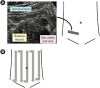
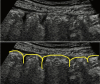
Intestinal ultrasound (IUS) is a promising modality for the management of inflammatory bowel disease (IBD) and has the potential to particularly contribute in monitoring disease activity, an advantage crucial for optimizing the therapeutic strategy. While many IBD physicians appreciate and are interested in the use of IUS for IBD, currently only a limited number of facilities can employ this examination in daily clinical practice. A lack of guidance is one of the major barriers to introducing this procedure. Standardized protocols and assessment criteria are needed such that IUS for IBD can be considered a feasible, reliable examination in clinical practice, and multicenter clinical studies can be conducted for further clinical evidence of the application of IUS in IBD for best patient care. In this article, we provide an overview of how to start IUS for IBD and introduce basic procedures. Furthermore, IUS images from our practice are provided as a color atlas for understanding sonographic findings and scoring systems. We anticipate this "first aid" article will be helpful to promote IUS for IBD in daily practice.
Keywords: Color atlas; Inflammatory bowel disease; Intestinal ultrasound; Sonographic parameters.
Conflict of interest statement
Miyoshi J received consultation and lecture fees from AbbVie GK, EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., and Takeda Pharmaceutical Co., Ltd. Morikubo H received a grant from Takeda Pharmaceutical Co., Ltd. Hisamatsu T received consultation and lecture fees from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; and received research grants from Alfresa Pharma Co., Ltd., EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, JIMRO Co., Ltd., Zeria Pharmaceutical Co., Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Inc., and Mochida Pharmaceutical Co., Ltd. Except for that, no potential conflict of interest relevant to this article was reported.
Figures




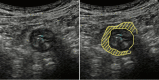

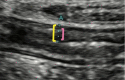




References
-
- Windsor JW, Kaplan GG. Evolving epidemiology of IBD. Curr Gastroenterol Rep. 2019;21:40. - PubMed
-
- Olén O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964-2014. Gut. 2020;69:453–461. - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. - PubMed
-
- Benitez JM, Meuwis MA, Reenaers C, Van Kemseke C, Meunier P, Louis E. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut. 2013;62:1806–1816. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

