Development of a Standardized, Laboratory Result-Based Hepatitis C Virus Clearance Cascade for Public Health Jurisdictions
- PMID: 37140162
- PMCID: PMC10851908
- DOI: 10.1177/00333549231170044
Development of a Standardized, Laboratory Result-Based Hepatitis C Virus Clearance Cascade for Public Health Jurisdictions
Keywords: continuity of patient care; data systems; hepatitis C; population health; public health surveillance.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

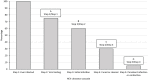
References
-
- US Department of Health and Human Services. Viral hepatitis: national strategic plan for the United States: a roadmap to elimination, 2021-2025. 2020. Accessed March 8, 2022. https://www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strateg...
-
- Sizemore LA, Wingate H, Black J, Wester CN. Tennessee’s 2017 hepatitis C virus continuum of cure. Abstract presented at: Council of State and Territorial Epidemiologists Annual Conference; June 4, 2019; Raleigh, North Carolina. Accessed March 29, 2022. https://cste.confex.com/cste/2019/meetingapp.cgi/Paper/11007
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

