Bacterial Decolonization for Prevention of Radiation Dermatitis: A Randomized Clinical Trial
- PMID: 37140904
- PMCID: PMC10160991
- DOI: 10.1001/jamaoncol.2023.0444
Bacterial Decolonization for Prevention of Radiation Dermatitis: A Randomized Clinical Trial
Abstract
Importance: Evidence-based approaches for the prevention of acute radiation dermatitis (ARD) are limited, and additional strategies are necessary to optimize care.
Objective: To determine the efficacy of bacterial decolonization (BD) to reduce ARD severity compared with standard of care.
Design, setting, and participants: This phase 2/3 randomized clinical trial was conducted from June 2019 to August 2021 with investigator blinding at an urban academic cancer center and enrolled patients with breast cancer or head and neck cancer receiving radiation therapy (RT) with curative intent. Analysis was performed on January 7, 2022.
Interventions: Intranasal mupirocin ointment twice daily and chlorhexidine body cleanser once daily for 5 days prior to RT and repeated for 5 days every 2 weeks through RT.
Main outcomes and measures: The primary outcome as planned prior to data collection was the development of grade 2 or higher ARD. Based on wide clinical variability of grade 2 ARD, this was refined to grade 2 ARD with moist desquamation (grade 2-MD).
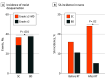
Results: Of 123 patients assessed for eligibility via convenience sampling, 3 were excluded, and 40 refused to participate, with 80 patients in our final volunteer sample. Of 77 patients with cancer (75 patients with breast cancer [97.4%] and 2 patients with head and neck cancer [2.6%]) who completed RT, 39 were randomly assigned BC, and 38 were randomly assigned standard of care; the mean (SD) age of the patients was 59.9 (11.9) years, and 75 (97.4%) were female. Most patients were Black (33.7% [n = 26]) or Hispanic (32.5% [n = 25]). Among patients with breast cancer and patients with head and neck cancer (N = 77), none of the 39 patients treated with BD and 9 of the 38 patients (23.7%) treated with standard of care developed ARD grade 2-MD or higher (P = .001). Similar results were observed among the 75 patients with breast cancer (ie, none treated with BD and 8 [21.6%] receiving standard of care developed ARD grade ≥2-MD; P = .002). The mean (SD) ARD grade was significantly lower for patients treated with BD (1.2 [0.7]) compared with patients receiving standard of care (1.6 [0.8]) (P = .02). Of the 39 patients randomly assigned to BD, 27 (69.2%) reported regimen adherence, and only 1 patient (2.5%) experienced an adverse event related to BD (ie, itch).
Conclusions and relevance: The results of this randomized clinical trial suggest that BD is effective for ARD prophylaxis, specifically for patients with breast cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT03883828.
Conflict of interest statement
Figures

Comment in
-
Bacterial Decolonization for Prevention of Radiation Dermatitis-Fragility Index Analysis.JAMA Oncol. 2024 Jan 1;10(1):142. doi: 10.1001/jamaoncol.2023.5007. JAMA Oncol. 2024. PMID: 37943535 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

