Intracellular trafficking of HLA-E and its regulation
- PMID: 37140910
- PMCID: PMC10165540
- DOI: 10.1084/jem.20221941
Intracellular trafficking of HLA-E and its regulation
Abstract
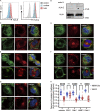
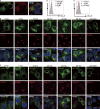
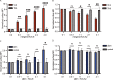
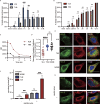
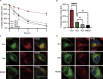
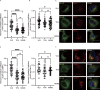
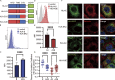
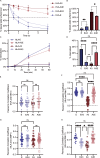
Interest in MHC-E-restricted CD8+ T cell responses has been aroused by the discovery of their efficacy in controlling simian immunodeficiency virus (SIV) infection in a vaccine model. The development of vaccines and immunotherapies utilizing human MHC-E (HLA-E)-restricted CD8+ T cell response requires an understanding of the pathway(s) of HLA-E transport and antigen presentation, which have not been clearly defined previously. We show here that, unlike classical HLA class I, which rapidly exits the endoplasmic reticulum (ER) after synthesis, HLA-E is largely retained because of a limited supply of high-affinity peptides, with further fine-tuning by its cytoplasmic tail. Once at the cell surface, HLA-E is unstable and is rapidly internalized. The cytoplasmic tail plays a crucial role in facilitating HLA-E internalization, which results in its enrichment in late and recycling endosomes. Our data reveal distinctive transport patterns and delicate regulatory mechanisms of HLA-E, which help to explain its unusual immunological functions.
© 2023 He et al.
Conflict of interest statement
Disclosures: A.J. McMichael reported grants from NIAID, BMGF, and the Chinese Academy of Medical Sciences–Oxford University Institute during the conduct of the study, personal fees from T-Cypher Bio, and “other” from E-Biotherapy outside the submitted work. In addition, A.J. McMichael, S. Brackenridge, and G.M. Gillespie are named inventors on a patent for HLA-E–binding epitope discovery issued, a patent for stabilizing peptide–HLA-E complexes issued, a patent for TCRs for anti-HIV T cells issued, a patent for antibodies that target HLA-E–host peptide complexes and uses thereof issued and a patent for monoclonal antibodies specific for cancer peptide–HLA-E pending. A.J. McMichael is also a named co-inventor of a patent for HLA-E binding, with royalties paid. He has ongoing discussions about forming a spin-out company, E-Biotherapy. This company is presently dormant. No other disclosures were reported.
Figures




References
-
- Barber, C., De Souza V.A., Paterson R.L., Martin-Urdiroz M., Mulakkal N.C., Srikannathasan V., Connolly M., Phillips G., Foong-Leong T., Pengelly R., et al. 2022. Structure-guided stabilization of pathogen-derived peptide-HLA-E complexes using non-natural amino acids conserves native TCR recognition. Eur. J. Immunol. 52:618–632. 10.1002/eji.202149745 - DOI - PMC - PubMed
-
- Borrego, F., Ulbrecht M., Weiss E.H., Coligan J.E., and Brooks A.G.. 1998. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J. Exp. Med. 187:813–818. 10.1084/jem.187.5.813 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

