Nanozyme-Based Robotics Approach for Targeting Fungal Infection
- PMID: 37141008
- PMCID: PMC10624647
- DOI: 10.1002/adma.202300320
Nanozyme-Based Robotics Approach for Targeting Fungal Infection
Abstract
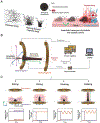
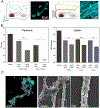
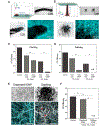
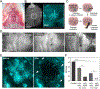
Fungal pathogens have been designated by the World Health Organization as microbial threats of the highest priority for global health. It remains a major challenge to improve antifungal efficacy at the site of infection while avoiding off-target effects, fungal spreading, and drug tolerance. Here, a nanozyme-based microrobotic platform is developed that directs localized catalysis to the infection site with microscale precision to achieve targeted and rapid fungal killing. Using electromagnetic field frequency modulation and fine-scale spatiotemporal control, structured iron oxide nanozyme assemblies are formed that display tunable dynamic shape transformation and catalysis activation. The catalytic activity varies depending on the motion, velocity, and shape providing controllable reactive oxygen species (ROS) generation. Unexpectedly, nanozyme assemblies bind avidly to fungal (Candida albicans) surfaces to enable concentrated accumulation and targeted ROS-mediated killing in situ. By exploiting these tunable properties and selective binding to fungi, localized antifungal activity is achieved using in vivo-like cell spheroid and animal tissue infection models. Structured nanozyme assemblies are directed to Candida-infected sites using programmable algorithms to perform precisely guided spatial targeting and on-site catalysis resulting in fungal eradication within 10 min. This nanozyme-based microrobotics approach provides a uniquely effective and targeted therapeutic modality for pathogen elimination at the infection site.
Keywords: Candida albicans; assemblies; biofilms; iron oxide; microrobots; mucosal.
© 2023 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Figures



References
-
- Vallabhaneni S, Mody RK, Walker T, Chiller T, Infect Dis Clin North Am. 2016, 30, 1–11. - PubMed
-
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC, Sci. Transl. Med. 2012, 4, 165rv13. - PubMed
-
- WHO, WHO fungal priority pathogens list to guide research, development and public health action, 2022, ISBN; 978-92-4-006024-1.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

