Comprehensive review of safety in Experimental Human Pneumococcal Challenge
- PMID: 37141259
- PMCID: PMC10159102
- DOI: 10.1371/journal.pone.0284399
Comprehensive review of safety in Experimental Human Pneumococcal Challenge
Abstract
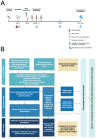
Introduction: Experimental Human Pneumococcal Challenge (EHPC) involves the controlled exposure of adults to a specific antibiotic-sensitive Streptococcus pneumoniae serotype, to induce nasopharyngeal colonisation for the purpose of vaccine research. The aims are to review comprehensively the safety profile of EHPC, explore the association between pneumococcal colonisation and frequency of safety review and describe the medical intervention required to undertake such studies.
Methods: A single-centre review of all EHPC studies performed 2011-2021. All recorded serious adverse events (SAE) in eligible studies are reported. An unblinded meta-analysis of collated anonymised individual patient data from eligible EHPC studies was undertaken to assess the association between experimental pneumococcal colonisation and the frequency of safety events following inoculation.
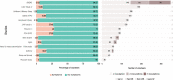
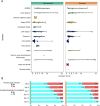
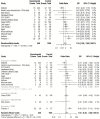
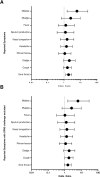
Results: In 1416 individuals (median age 21, IQR 20-25), 1663 experimental pneumococcal inoculations were performed. No pneumococcal-related SAE have occurred. 214 safety review events were identified with 182 (12.85%) participants presenting with symptoms potentially in keeping with pneumococcal infection, predominantly in pneumococcal colonised individuals (colonised = 96/658, non-colonised = 86/1005, OR 1.81 (95% CI 1.28-2.56, P = <0.001). The majority were mild (pneumococcal group = 72.7% [120/165 reported symptoms], non-pneumococcal = 86.7% [124/143 reported symptoms]). 1.6% (23/1416) required antibiotics for safety.
Discussion: No SAEs were identified directly relating to pneumococcal inoculation. Safety review for symptoms was infrequent but occurred more in experimentally colonised participants. Most symptoms were mild and resolved with conservative management. A small minority required antibiotics, notably those serotype 3 inoculated.
Conclusion: Outpatient human pneumococcal challenge can be conducted safely with appropriate levels of safety monitoring procedures in place.
Copyright: © 2023 Robinson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organisation. Human Challenge Trials for Vaccine Development: regulatory considerations. Available at https://www.who.int/biologicals/expert_committee/Human_challenge_Trials_... World Health Organisation;2016. (Accessed 15/08/22).
-
- Medicines and Healthcare products Regulatory Agency. Good Clinical Practice Guide. United Kingdom: TSO; 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

