Adoption is not associated with immunological and virological outcomes in children with perinatally acquired HIV infection in the Netherlands
- PMID: 37141310
- PMCID: PMC10159147
- DOI: 10.1371/journal.pone.0284395
Adoption is not associated with immunological and virological outcomes in children with perinatally acquired HIV infection in the Netherlands
Abstract
Objectives: To provide an overview of the demographics, treatment characteristics and long-term outcomes of children with perinatal HIV-1 infection (PHIV) living in the Netherlands (NL) and to specifically investigate whether outcomes differ by children's adoption status.
Design: A prospective population-based open cohort including children with PHIV in NL.
Methods: We included children with PHIV who had entered HIV care in NL since 2007, in view of a sharp increase in the number of adopted children with PHIV since that year. We compared the proportion with virologic suppression and CD4+T-cell count over time between the following groups of children with PHIV: adopted and born outside NL, non-adopted born in NL, and non-adopted born outside NL, using generalized estimating equations and linear mixed effects models, respectively. To account for the variation in cohort inclusion, we analyzed data of children exposed to at least one year of antiretroviral therapy (ART).
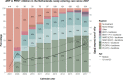
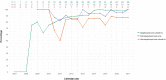
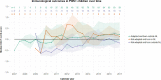
Results: We included 148 children (827.5 person-years of follow-up, 72% adopted, age at start care in NL 2.4 (0.5-5.3)). Under-18 mortality was zero. Over the years, a boosted PI-based regimen was most often prescribed. The use of integrase inhibitors increased since 2015. Non-adopted children born in NL were less likely to achieve virological suppression compared to adopted children (OR 0.66, 95%CI 0.51-0.86, p = 0.001), which disappeared after excluding one child with suspected treatment nonadherence (OR 0.85, 95%CI 0.57-1.25, p = 0.400). CD4+T-cell Z-score trajectories were not significantly different between groups.
Conclusions: Despite considerable and increasing diversity of the population of children with PHIV in NL, geographical origin and adoption status do not seem to pose important challenges in achieving good immunological and virological outcomes.
Copyright: © 2023 Van Den Hof et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
MH, CS, AvR, TW, SG, ES, KvA, FW and DP and reported no conflicts of interest. PF receives funding from PREPARE Europe (EU FP7 grant no 602525). HS received a small grant from Gilead. PR through his institution has received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals Inc, Merck & Co and ViiV Healthcare, and has served on scientific advisory boards for Gilead Sciences, ViiV Healthcare, Merck & Co, Teva pharmaceutical industries, for which honoraria were all paid to his institution. None related to the current manuscript. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Boer K, Smit C, van der Flier M, de Wolf F, group Acs. The comparison of the performance of two screening strategies identifying newly-diagnosed HIV during pregnancy. Eur J Public Health. 2011;21(5):632–7. - PubMed
-
- van Sighem A.I.BTS, Wit F.W.N.M., Smit C., Matser A., Reiss P. Monitoring Report 2018. Human Immunodeficiency Virus (HIV) Infection in the Netherlands. Amsterdam: Stichting HIV Monitoring; 2018.
-
- Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

