Weight loss efficiency and safety of tirzepatide: A Systematic review
- PMID: 37141329
- PMCID: PMC10159347
- DOI: 10.1371/journal.pone.0285197
Weight loss efficiency and safety of tirzepatide: A Systematic review
Abstract
Objective: Tirzeptide is a novel glucagon-like peptide-1 receptor (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) drug, which shows good efficiency for weight loss. Therefore, we aim to investigate the efficacy and safety of tirzepatide for weight loss in type 2 diabetes mellitus (T2DM) and obesity patients in this meta-analysis study.
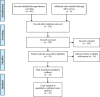
Methods: Cochrane Library, PubMed, Embase, Clinical Trials, and Web of Science were searched from inception to October 5, 2022. All randomized controlled trials (RCTs) were included. The odds ratio (OR) was calculated using fixed-effects or random-effects models by Review Manager 5.3 software.
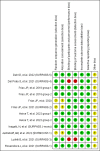
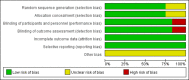
Results: In total, ten studies (12 reports) involving 9,873 patients were identified. A significant loss body weight in the tirzepatide group versus the placebo by -9.81 kg (95% CI (-12.09, -7.52), GLP-1 RAs by -1.05 kg (95% CI (-1.48, -0.63), and insulin by -1.93 kg (95% CI (-2.81, -1.05), respectively. In sub-analysis, the body weight of patients was significantly reduced in three tirzepatide doses (5 mg, 10 mg, and 15 mg) when compared with those of the placebo/GLP-1 RA/insulin. In terms of safety, the incidence of any adverse events and adverse events leading to study drug discontinuation was higher in the tirzepatide group, but the incidence of serious adverse events and hypoglycaemia was lower. Additionally, the gastrointestinal adverse events (including diarrhea, nausea, vomiting and decreased appetite) of tirzepatide were higher than those of placebo/basal insulin, but similar to GLP-1 RAs.
Conclusion: In conclusion, tirzeptide can significantly reduce the weight of T2DM and patient with obesity, and it is a potential therapeutic regimen for weight-loss, but we need to be vigilant about its gastrointestinal reaction.
Copyright: © 2023 Lin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

