The Temporal Relationship Between the Coronavirus Disease 2019 (COVID-19) Pandemic and Preterm Birth
- PMID: 37141586
- PMCID: PMC10440253
- DOI: 10.1097/AOG.0000000000005171
The Temporal Relationship Between the Coronavirus Disease 2019 (COVID-19) Pandemic and Preterm Birth
Abstract
Objective: To evaluate whether preterm birth rates changed in relation to the onset of the coronavirus disease 2019 (COVID-19) pandemic and whether any change depended on socioeconomic status.
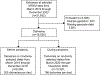
Methods: This is an observational cohort study of pregnant individuals with a singleton gestation who delivered in the years 2019 and 2020 at 1 of 16 U.S. hospitals of the Maternal-Fetal Medicine Units Network. The frequency of preterm birth for those who delivered before the onset of the COVID-19 pandemic (ie, in 2019) was compared with that of those who delivered after its onset (ie, in 2020). Interaction analyses were performed for people of different individual- and community-level socioeconomic characteristics (ie, race and ethnicity, insurance status, Social Vulnerability Index (SVI) of a person's residence).
Results: During 2019 and 2020, 18,526 individuals met inclusion criteria. The chance of preterm birth before the COVID-19 pandemic was similar to that after the onset of the pandemic (11.7% vs 12.5%, adjusted relative risk 0.94, 95% CI 0.86-1.03). In interaction analyses, race and ethnicity, insurance status, and the SVI did not modify the association between the epoch and the chance of preterm birth before 37 weeks of gestation (all interaction P >.05).
Conclusion: There was no statistically significant difference in preterm birth rates in relation to the COVID-19 pandemic onset. This lack of association was largely independent of socioeconomic indicators such as race and ethnicity, insurance status, or SVI of the residential community in which an individual lived.
Copyright © 2023 by the American College of Obstetricians and Gynecologists. Published by Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Financial Disclosure: Torri D. Metz disclosed receiving payment from UpToDate (royalties for two topics on trial of labor after cesarean). Money was paid to her institution from Gestvision (site PI for preeclampsia point-of-care test; institution received money to conduct study [ended August 2020])and Pfizer (site PI for Phase III respiratory syncytial virus [RSV] vaccine trial, institution received money to conduct study). She is a member of medical advisory board for Pfizer and site PI for a COVID-19 vaccination trial in pregnancy Society for Maternal-Fetal Medicine Board of Directors (unpaid). Brenna L. Hughes disclosed receiving payment from Merck, UpToDate, and Johns Hopkins. The other authors did not report any potential conflicts of interest.
Figures
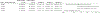
References
Publication types
MeSH terms
Grants and funding
- U10 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- UG1 HD087230/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD087192/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

