Organism-wide, cell-type-specific secretome mapping of exercise training in mice
- PMID: 37141889
- PMCID: PMC10524249
- DOI: 10.1016/j.cmet.2023.04.011
Organism-wide, cell-type-specific secretome mapping of exercise training in mice
Abstract
There is a significant interest in identifying blood-borne factors that mediate tissue crosstalk and function as molecular effectors of physical activity. Although past studies have focused on an individual molecule or cell type, the organism-wide secretome response to physical activity has not been evaluated. Here, we use a cell-type-specific proteomic approach to generate a 21-cell-type, 10-tissue map of exercise training-regulated secretomes in mice. Our dataset identifies >200 exercise training-regulated cell-type-secreted protein pairs, the majority of which have not been previously reported. Pdgfra-cre-labeled secretomes were the most responsive to exercise training. Finally, we show anti-obesity, anti-diabetic, and exercise performance-enhancing activities for proteoforms of intracellular carboxylesterases whose secretion from the liver is induced by exercise training.
Keywords: CES2; cell type; energy metabolism; exercise; exercise performance; hepatokine; obesity; secretome; tissue crosstalk.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Stanford University has filed a provisional patent on extracellular CES2 proteins and methods of use.
Figures



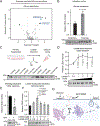

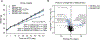
References
-
- Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, et al. (2017). The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet 390, 2643–2654. 10.1016/S0140-6736(17)31634-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

