Towards optimizing single pulse electrical stimulation: High current intensity, short pulse width stimulation most effectively elicits evoked potentials
- PMID: 37141936
- PMCID: PMC10330807
- DOI: 10.1016/j.brs.2023.04.023
Towards optimizing single pulse electrical stimulation: High current intensity, short pulse width stimulation most effectively elicits evoked potentials
Abstract
Background: While single pulse electrical stimulation (SPES) is increasingly used to study effective connectivity, the effects of varying stimulation parameters on the resulting cortico-cortical evoked potentials (CCEPs) have not been systematically explored.
Objective: We sought to understand the interacting effects of stimulation pulse width, current intensity, and charge on CCEPs through an extensive testing of this parameter space and analysis of several response metrics.
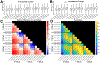
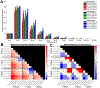
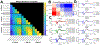
Methods: We conducted SPES in 11 patients undergoing intracranial EEG monitoring using five combinations of current intensity (1.5, 2.0, 3.0, 5.0, and 7.5 mA) and pulse width at each of three charges (0.750, 1.125, and 1.500 μC/phase) to study how CCEP amplitude, distribution, latency, morphology, and stimulus artifact amplitude vary with each parameter.
Results: Stimulations with a greater charge or a greater current intensity and shorter pulse width at a given charge generally resulted in greater CCEP amplitudes and spatial distributions, shorter latencies, and increased waveform correlation. These effects interacted such that stimulations with the lowest charge and highest current intensities resulted in greater response amplitudes and spatial distributions than stimulations with the highest charge and lowest current intensities. Stimulus artifact amplitude increased with charge, but this could be mitigated by using shorter pulse widths.
Conclusions: Our results indicate that individual combinations of current intensity and pulse width, in addition to charge, are important determinants of CCEP magnitude, morphology, and spatial extent. Together, these findings suggest that high current intensity, short pulse width stimulations are optimal SPES settings for eliciting strong and consistent responses while minimizing charge.
Keywords: Cortico-cortical evoked potential; Effective connectivity; Intracranial EEG; Single pulse electrical stimulation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

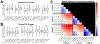
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

