Comparison of first-line radiosurgery for small-cell and non-small cell lung cancer brain metastases (CROSS-FIRE)
- PMID: 37142267
- PMCID: PMC10407696
- DOI: 10.1093/jnci/djad073
Comparison of first-line radiosurgery for small-cell and non-small cell lung cancer brain metastases (CROSS-FIRE)
Abstract
Introduction: Historical reservations regarding stereotactic radiosurgery (SRS) for small-cell lung cancer (SCLC) brain metastases include concerns for short-interval and diffuse central nervous system (CNS) progression, poor prognoses, and increased neurological mortality specific to SCLC histology. We compared SRS outcomes for SCLC and non-small cell lung cancer (NSCLC) where SRS is well established.
Methods: Multicenter first-line SRS outcomes for SCLC and NSCLC from 2000 to 2022 were retrospectively collected (n = 892 SCLC, n = 4785 NSCLC). Data from the prospective Japanese Leksell Gamma Knife Society (JLGK0901) clinical trial of first-line SRS were analyzed as a comparison cohort (n = 98 SCLC, n = 814 NSCLC). Overall survival (OS) and CNS progression were analyzed using Cox proportional hazard and Fine-Gray models, respectively, with multivariable adjustment for cofactors including age, sex, performance status, year, extracranial disease status, and brain metastasis number and volume. Mutation-stratified analyses were performed in propensity score-matched retrospective cohorts of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) positive NSCLC, mutation-negative NSCLC, and SCLC.
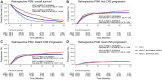
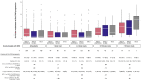
Results: OS was superior for patients with NSCLC compared to SCLC in the retrospective dataset (median OS = 10.5 vs 8.6 months; P < .001) and in the JLGK0901 dataset. Hazard estimates for first CNS progression favoring NSCLC were similar in both datasets but reached statistical significance in the retrospective dataset only (multivariable hazard ratio = 0.82, 95% confidence interval = 0.73 to 0.92, P = .001). In the propensity score-matched cohorts, there were continued OS advantages for NSCLC patients (median OS = 23.7 [EGFR and ALK positive NSCLC] vs 13.6 [mutation-negative NSCLC] vs 10.4 months [SCLC], pairwise P values < 0.001), but no statistically significant differences in CNS progression were observed in the matched cohorts. Neurological mortality and number of lesions at CNS progression were similar for NSCLC and SCLC patients. Leptomeningeal progression was increased in patients with NSCLC compared to SCLC in the retrospective dataset only (multivariable hazard ratio = 1.61, 95% confidence interval = 1.14 to 2.26, P = .007).
Conclusions: After SRS, SCLC histology was associated with shorter OS compared to NSCLC. CNS progression occurred earlier in SCLC patients overall but was similar in patients matched on baseline factors. SCLC was not associated with increased neurological mortality, number of lesions at CNS progression, or leptomeningeal progression compared to NSCLC. These findings may better inform clinical expectations and individualized decision making regarding SRS for SCLC patients.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Chad Rusthoven discloses unpaid committee panel participation for National Comprehensive Cancer Network (NCCN CNS guideline panel, NCCN Small Cell Lung Cancer guideline panel, American Society for Radiation Oncology (ASTRO) Brain Metastases guideline panel; honorarium from Sonofi. Rodney Wegner discloses a research grant from Elekta. Nicolaus Andratschke discloses grants from Swiss National Fund Swiss Cancer League, University of Zurich Funds Swiss Private Foundations, ViewRay Inc, Brainlab AG; consulting fees from ViewRay Inc, Brainlab AG, AstraZeneca; honoraria from ViewRay Inc, Brainlab AG, AstraZeneca; travel support from ViewRay Inc, Brainlab AG, AstraZeneca; participation on a data safety monitoring board or advisory board for AstraZeneca; leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid for Swiss Association of Clinical Trials EORTC, ESTRO, Global Harmonization Group; stock or stock optins for Moderna Inc, Idorsia AG. Denise Bernhardt discloses payment or honoraria from AstraZeneca, Novocure; participation on a data safety monitoring board or advisory board for Novocure GmbH; stock or stock options for Gilead Science. Michael Chan discloses honoraria from Monteris Inc. Christopher Cifarelli discloses honoraria from Carl Zeiss Meditec AG. Jurgen Debus discloses grants from Viewray Inc, CRI—The Clinical Research Institute GmbH, Accuray International Sari, RaySearch Laboratories AB, Vision RT Limited, Merck Serono GmbH, Astellas Pharma GmbH, Astra Zeneca GmbH, Siemens Healthcare GmbH, Solution Akademie GmbH, Ergomed PLC Surrey Research Park, Quintiles GmbH, Pharmaceutical Research Associates GmbH, Boehringer Ingelheim Pharma GmbH & CoKG, PTW-Freiburg Dr Pychlau GmbH, Nanobiotix S.A., Accuray Incorporated. Ramie El SHafi discloses grants from Accuray Inc, Ruprecht-Karls Universität Heidelberg; honraria from AstraZeneca GmbH, Accuray Inc, Bristol Myers Squibb GmbH & Co, Novocure GmbH, Merck KGaA, Takeda GmbH; travel support from AstraZeneca GmbH, Accuray Inc, Bristol Myers Squibb GmbH & Co, Novocure GmbH, Merck KGaA, Takeda GmbH; stock or stock options from Takeda GmbH. Jona Hattangadi-Gluth discloses grants from the NIH/NCI; honorarium from Aptitude Health. L. Dade Lunsford discloses participation on a data safety monitoring board or advisory board for DSMB Insightec; stock or stock options AB Elekta. Joshua Palme discloses grants from the NIH and Genentech; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Varian Medical Systems, Novocure, ICOTEC; travel support from Varian, ICOTEC; participation on a data safety monitoring board or advisory board from Novocure. Daniel Trifiletti discloses consulting fees from Boston Scientific; board membership for International Stereotactic Radiosurgery Society. Lia Halasz discloses clinical trial funding from BioMimetix. Paul Brown discloses honorarium from UpToDate.com. Stephanie Combs discloses consulting fees from Icotec AG, HMG Systems Engineering, Bristol Myers Squibb; speaking fees and travel support from Roche, BMS, Brainlab, AstraZeneca, Accuray, Dr Sennewald, Daiichi Sankyo, Elekta, Medac, Med Update GmbH, Carl Zeiss Meditec AG; leadership on NOA board, DEGRO board.
Figures


References
-
- Nabors LB, Portnow J, Ahluwalia M, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18(11):1537-1570. - PubMed
-
- Le Rhun E, Guckenberger M, Smits M, et al.; EANO Executive Board and ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. EANO–ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. ⋆. Ann Oncol. 2021;32(11):1332-1347. - PubMed
-
- Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. Oxford University Press US; 2022. - PubMed
-
- Wu Y-L, Planchard D, Lu S, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO–ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171-210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

