Study protocol of an open-label proof-of-concept trial examining the safety and clinical efficacy of psilocybin-assisted therapy for veterans with PTSD
- PMID: 37142308
- PMCID: PMC10163450
- DOI: 10.1136/bmjopen-2022-068884
Study protocol of an open-label proof-of-concept trial examining the safety and clinical efficacy of psilocybin-assisted therapy for veterans with PTSD
Abstract
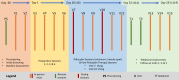
Introduction: Psilocybin-assisted therapy has shown significant promise in treating the cluster of mood and anxiety symptoms that comprise post-traumatic stress disorder (PTSD) but has yet to be tested specifically in this condition. Furthermore, current pharmacological and psychotherapeutic treatments for PTSD are difficult to tolerate and limited in efficacy, especially in the US Military Veteran (USMV) population. This open-label pilot study will examine the safety and efficacy of two psilocybin administration sessions (15 mg and 25 mg), combined with psychotherapy, among USMVs with severe, treatment resistant PTSD.
Methods and analysis: We will recruit 15 USMVs with severe, treatment resistant PTSD. Participants will receive one low dose (15 mg) and one moderate/high dose (25 mg) of psilocybin in conjunction with preparatory and post-psilocybin therapy sessions. The primary safety outcome will be the type, severity and frequency of adverse events and suicidal ideation/behaviour, as measured by the Columbia Suicide Severity Rating Scale. The primary outcome measure for PTSD will be the Clinician Administered PTSD Scale-5. The primary endpoint will be 1 month following the second psilocybin administration session, and the total follow-up time will be 6 months.
Ethics and dissemination: All participants will be required to provide written informed consent. The trial has been authorised by the Ohio State University Institutional Review Board (study number: 2022H0280). Dissemination of results will occur via a peer-reviewed publication and other relevant media.
Trial registration number: NCT05554094.
Keywords: Adult psychiatry; CLINICAL PHARMACOLOGY; Clinical trials; MENTAL HEALTH; PSYCHIATRY.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AKD and RLL are board members of Source Research Foundation. This organisation was not involved in the design/execution of this study or the interpretation or communication of findings. AKD is a lead trainer at Fluence.
Figures
Similar articles
-
Psilocybin-assisted massed cognitive processing therapy for chronic posttraumatic stress disorder: Protocol for an open-label pilot feasibility trial.PLoS One. 2025 Jan 17;20(1):e0313741. doi: 10.1371/journal.pone.0313741. eCollection 2025. PLoS One. 2025. PMID: 39823496 Free PMC article.
-
3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial.Lancet Psychiatry. 2018 Jun;5(6):486-497. doi: 10.1016/S2215-0366(18)30135-4. Epub 2018 May 1. Lancet Psychiatry. 2018. PMID: 29728331 Clinical Trial.
-
Psilocybin-Assisted suppoRtive psychoTherapy IN the treatment of prolonged Grief (PARTING) trial: protocol for an open-label pilot trial for cancer-related bereavement.BMJ Open. 2025 Apr 15;15(4):e095992. doi: 10.1136/bmjopen-2024-095992. BMJ Open. 2025. PMID: 40233965 Free PMC article.
-
Psilocybin for Trauma-Related Disorders.Curr Top Behav Neurosci. 2022;56:319-332. doi: 10.1007/7854_2022_366. Curr Top Behav Neurosci. 2022. PMID: 35711024 Review.
-
Psilocybin and MDMA for the treatment of trauma-related psychopathology.Int Rev Psychiatry. 2021 May;33(3):229-249. doi: 10.1080/09540261.2021.1919062. Epub 2021 Jun 14. Int Rev Psychiatry. 2021. PMID: 34121583 Review.
Cited by
-
Effects of discontinuation of serotonergic antidepressants prior to psilocybin therapy versus escitalopram for major depression.J Psychopharmacol. 2024 May;38(5):458-470. doi: 10.1177/02698811241237870. Epub 2024 Mar 22. J Psychopharmacol. 2024. PMID: 38520045 Free PMC article. Clinical Trial.
-
Novel Pharmacological Targets of Post-Traumatic Stress Disorders.Life (Basel). 2023 Aug 11;13(8):1731. doi: 10.3390/life13081731. Life (Basel). 2023. PMID: 37629588 Free PMC article. Review.
-
The Emergence of Psilocybin in Psychiatry and Neuroscience.Pharmaceuticals (Basel). 2025 Apr 9;18(4):555. doi: 10.3390/ph18040555. Pharmaceuticals (Basel). 2025. PMID: 40283990 Free PMC article. Review.
-
Exploring the Therapeutic Effects of Psychedelics Administered to Military Veterans in Naturalistic Retreat Settings.Brain Behav. 2025 Jul;15(7):e70660. doi: 10.1002/brb3.70660. Brain Behav. 2025. PMID: 40619953 Free PMC article.
-
Psilocybin as Transformative Fast-Acting Antidepressant: Pharmacological Properties and Molecular Mechanisms.Fundam Clin Pharmacol. 2025 Aug;39(4):e70038. doi: 10.1111/fcp.70038. Fundam Clin Pharmacol. 2025. PMID: 40670864 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical