Validation of immunoassays for the Chlamydia trachomatis antigen Pgp3 using a chimeric monoclonal antibody
- PMID: 37142607
- PMCID: PMC10160048
- DOI: 10.1038/s41598-023-33834-4
Validation of immunoassays for the Chlamydia trachomatis antigen Pgp3 using a chimeric monoclonal antibody
Abstract
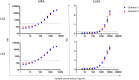
Seroepidemiology, or measuring antibodies to pathogens to estimate population-level exposure, can provide useful public health data. The tests used, however, often lack sufficient validation data due to absence of a gold standard. For many pathogens, serum antibodies can be detected long after resolution of infection, but infection status is often used as a gold standard for antibody positivity. To ensure that recently developed antibody tests for seroepidemiology of Chlamydia trachomatis (Ct), the causative agent of urogenital chlamydia and the blinding eye disease trachoma, have high performance, we generated a chimeric antibody to the immunodominant Ct antigen Pgp3. Two clones were selected to evaluate the test performance of three assays to measure antibodies to Pgp3: multiplex bead assay (MBA), enzyme-linked immunosorbent assay (ELISA), and lateral flow assay (LFA). Overall, each assay demonstrated high accuracy and precision when tested using either clone, and the clones were stable when stored at - 20 °C and 4 °C for almost 2 years. The limit of detection was similar for MBA and LFA, but almost a log-fold higher (i.e. less sensitive) using ELISA. Overall, the chimeric antibodies represent stable control reagents for tests with robust performance and will facilitate deployment of these tests to other laboratories.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

