Raman spectroscopy and topological machine learning for cancer grading
- PMID: 37142690
- PMCID: PMC10160071
- DOI: 10.1038/s41598-023-34457-5
Raman spectroscopy and topological machine learning for cancer grading
Abstract
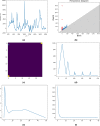
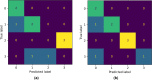
In the last decade, Raman Spectroscopy is establishing itself as a highly promising technique for the classification of tumour tissues as it allows to obtain the biochemical maps of the tissues under investigation, making it possible to observe changes among different tissues in terms of biochemical constituents (proteins, lipid structures, DNA, vitamins, and so on). In this paper, we aim to show that techniques emerging from the cross-fertilization of persistent homology and machine learning can support the classification of Raman spectra extracted from cancerous tissues for tumour grading. In more detail, topological features of Raman spectra and machine learning classifiers are trained in combination as an automatic classification pipeline in order to select the best-performing pair. The case study is the grading of chondrosarcoma in four classes: cross and leave-one-patient-out validations have been used to assess the classification accuracy of the method. The binary classification achieves a validation accuracy of 81% and a test accuracy of 90%. Moreover, the test dataset has been collected at a different time and with different equipment. Such results are achieved by a support vector classifier trained with the Betti Curve representation of the topological features extracted from the Raman spectra, and are excellent compared with the existing literature. The added value of such results is that the model for the prediction of the chondrosarcoma grading could easily be implemented in clinical practice, possibly integrated into the acquisition system.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

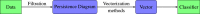

References
-
- Long D. The Raman Effect. Wiley; 2002.
-
- Bergholt M, et al. Raman endoscopy for objective diagnosis for early cancer in the gastrointestinal system. J. Gastroint. Dig. Syst. 2013;S1:008.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

