EZH2 inhibition remodels the inflammatory senescence-associated secretory phenotype to potentiate pancreatic cancer immune surveillance
- PMID: 37142692
- PMCID: PMC10516132
- DOI: 10.1038/s43018-023-00553-8
EZH2 inhibition remodels the inflammatory senescence-associated secretory phenotype to potentiate pancreatic cancer immune surveillance
Abstract
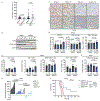
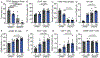
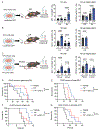
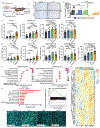
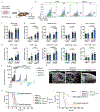
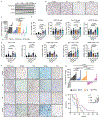
Immunotherapies that produce durable responses in some malignancies have failed in pancreatic ductal adenocarcinoma (PDAC) due to rampant immune suppression and poor tumor immunogenicity. We and others have demonstrated that induction of the senescence-associated secretory phenotype (SASP) can be an effective approach to activate anti-tumor natural killer (NK) cell and T cell immunity. In the present study, we found that the pancreas tumor microenvironment suppresses NK cell and T cell surveillance after therapy-induced senescence through enhancer of zeste homolog 2 (EZH2)-mediated epigenetic repression of proinflammatory SASP genes. EZH2 blockade stimulated production of SASP chemokines CCL2 and CXCL9/10, leading to enhanced NK cell and T cell infiltration and PDAC eradication in mouse models. EZH2 activity was also associated with suppression of chemokine signaling and cytotoxic lymphocytes and reduced survival in patients with PDAC. These results demonstrate that EZH2 represses the proinflammatory SASP and that EZH2 inhibition combined with senescence-inducing therapy could be a powerful means to achieve immune-mediated tumor control in PDAC.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING INTERESTS
S.W.L. is a founder and member of the scientific advisory board of Blueprint Medicines, Mirimus Inc., ORIC Pharmaceuticals, Geras Bio, and Faeth Therapeutics, and is on the scientific advisory board of PMV Pharmaceuticals. M.R. is a consultant for Boehringer Ingelheim. L.C. and M.R. have filed a U.S. patent application (Ser. No. 63/249,716) related to this work. The other authors declare no competing interests.
Figures











Comment in
-
Combining EZH2 inhibition with senescence induction helps immune cells fight pancreatic cancer.Nat Rev Immunol. 2023 Jul;23(7):411. doi: 10.1038/s41577-023-00898-2. Nat Rev Immunol. 2023. PMID: 37277561 No abstract available.
-
EZH2i unlocks PDAC immune surveillance.Nat Cancer. 2023 Jun;4(6):781-783. doi: 10.1038/s43018-023-00562-7. Nat Cancer. 2023. PMID: 37369835 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

