Features and Long-Term Outcomes of Stage IV Melanoma Patients Achieving Complete Response Under Anti-PD-1-Based Immunotherapy
- PMID: 37142875
- PMCID: PMC10195722
- DOI: 10.1007/s40257-023-00775-7
Features and Long-Term Outcomes of Stage IV Melanoma Patients Achieving Complete Response Under Anti-PD-1-Based Immunotherapy
Abstract
Background: Immune checkpoint inhibition (ICI) has changed the melanoma treatment spectrum. Few studies have examined the characteristics and long-term outcomes of patients achieving complete response (CR) under ICI.
Materials and methods: We evaluated patients with unresectable stage IV melanoma treated with first-line ICI. The characteristics of those achieving CR were compared with those not achieving CR. Progression-free survival (PFS) and overall survival (OS) were assessed. Late-onset toxicities, response to second-line treatment, the prognostic value of clinicopathologic features, and blood markers were examined.
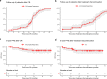
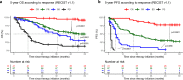
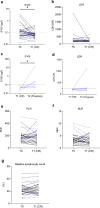
Results: A total of 265 patients were included; 41 (15.5%) achieved CR, while 224 (84.5%) had progressive disease, stable disease, or partial response. At the therapy start, those who had CR were more likely to be older than 65 years of age (p = 0.013), have a platelet-to-lymphocyte ratio below 213 (p = 0.036), and have lower lactate dehydrogenase levels (p = 0.008) than those not achieving a CR. For those who discontinued therapy after CR, the median follow-up time after CR was 56 months (interquartile range [IQR] 52-58) and the median time from CR to therapy end was 10 months (IQR 1-17). Five-year PFS after CR was 79% and 5-year OS was 83%. Most complete responders had a normalization of S100 at the time of CR (p < 0.001). In simple Cox regression analysis, age below 77 years at CR (p = 0.04) was associated with better prognosis after CR. Eight patients received second-line ICI; disease control was seen in 63%. Late immune-related toxicities occurred in 25% of patients, most being cutaneous immune-related toxicities.
Conclusions: Response, according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, is, until now, the most important prognostic factor, and CR is a valid surrogate marker for long-term survival in patients treated with ICI. Our results highlight the importance of investigating the optimal therapy duration in complete responders.
© 2023. The Author(s).
Conflict of interest statement
Ulrike Leiter reports research support from MSD; consulting fees and honoraria from Sun Pharma, Sanofi (personal and institutional), MSD (personal and institutional), Novartis, Roche, and Almirall Hermal; support for attending meetings from Sun Pharma; and participation on a Data Safety Monitoring Board or Advisory Board from Sun Pharma, Sanofi, MSD, Novartis, Roche, and Almirall Hermal, outside the submitted work. Ioannis Thomas reports honoraria for talks or advisory boards from BMS and Pierre Fabre; and research funding (institutional) from BMS, Pfizer, MSD, Amgen, ARGN-X, LEO, Novartis, UCB, 4SC, AstraZeneca, BioNTech, Genentech, Roche, Biotech, CureVac, HUYA, Incyte, Idera, Iovance, InflaRx, Cerpass, Kartos, Nektar, Philogen, Pierre Fabre, Regeneron, Replimune, and Sanofi. Heike Niessner reports institutional funding from Novartis and Pierre Fabre outside the submitted work. Tobias Sinnberg reports institutional funding from Novartis and Pierre Fabre outside the submitted work. Andrea Forschner served as a consultant to Roche, Novartis, MSD, BMS, and Pierre Fabre; received travel support from Roche, Novartis, BMS, and Pierre Fabre; received speaker fees from Roche, Novartis, BMS, MSD, and CeGaT; and reports institutional research grants from BMS Stiftung Immunonkologie. Lukas Flatz reports grants or contracts from Hookipa Pharma, SAKK/Immunophotonics, DFG Grant (Deutsche Forschungsgemeinschaft), Philogen, and Mundipharma; consulting fees from Philogen, Sanofi, Novartis, and BMS; participation on the Data Safety Board, University of Basel; and stocks or stock options from Hookipa Pharma, outside the submitted work. Teresa Amaral reports personal honoraria from BMS, CeCaVa, Novartis, and Pierre Fabre; institutional financial support from iFIT, NeraCare, Novartis, Sanofi, and SkylineDx, and an institutional research grant from Novartis, outside the submitted work. Eftychia Chatziioannou, Ulrike Keim, Olivia Seeber, Andreas Meiwes, Isabell Boessenecker, Stephanie Sanchez Gonzalez, and Francisco Merraz Torres have no relationships to disclose.
Figures

References
-
- McDermott D, Lebbé C, Hodi FS, Maio M, Weber JS, Wolchok JD, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40(9):1056–1064. - PubMed
-
- Ribas A, Hodi FS, Kefford R, Hamid O, Daud A, Wolchok JD, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL) J Clin Oncol. 2014;32(15 Suppl):LBA9000-LBA.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

