Tumor microenvironmental modification by the current target therapy for head and neck squamous cell carcinoma
- PMID: 37143088
- PMCID: PMC10161653
- DOI: 10.1186/s13046-023-02691-4
Tumor microenvironmental modification by the current target therapy for head and neck squamous cell carcinoma
Abstract
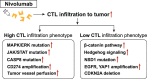
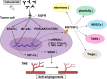
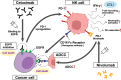
Current clinical and observational evidence supports the EXTREME regimen as one of the standards of care for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) followed by the administration of immune checkpoint inhibitors (ICIs). In addition to the inhibition of the epidermal growth factor receptor (EGFR) pathway, cetuximab-mediated EGFR blockade has been shown to modulate tumor microenvironment (TME) characteristics, such as antibody-dependent cellular cytotoxicity (ADCC) activity, cytotoxic T-lymphocyte (CTL) infiltration into the tumor, anti-angiogenesis activity, and cytokine secretion via associated natural killer (NK) cells, etc.. On the other hand, there are reports that nivolumab affects the TME via Programmed cell death 1 (PD-1) inhibition, Interleukin-10 upregulation via T-cells, myeloid-derived suppressor cell-mediated immune escape induction, and tumor vessel perfusion by promoting CD8 + T-cell accumulation and Interferon-γ production in treatment-sensitive tumor cells. Actually, nivolumab administration can give T cells in the TME both immune superiority and inferiority. HNSCC treatment using cetuximab increases the frequency of FoxP3 + intratumoral effector regulatory T cells (Tregs) expressing CTL associated antigen (CTLA)-4, and targeting CTLA-4 + Tregs using ipilimumab restores the cytolytic function of NK cells, which mediate ADCC activity. Treg-mediated immune suppression also contributes to clinical response to cetuximab treatment, suggesting the possibility of the addition of ipilimumab or the use of other Treg ablation strategies to promote antitumor immunity. Moreover, also in hyper progression disease (HPD), intratumoral frequency of FoxP3 + effector Tregs expressing CTLA-4 is increased. Therefore, combination treatment with cetuximab plus anti-CTLA-4 antibody ipilimumab for HNSCC and this combination therapy after nivolumab administration for HPD may be expected to result in a higher tumor-control response. Based on the above evidence, we here suggest the efficacy of using these therapeutic strategies for patients with local-advanced, recurrent, and metastatic HNSCC and patients who do not respond well to nivolumab administration.
Keywords: CTLA-4; Cetuximab; Head and neck squamous cell carcinoma; Immune checkpoint inhibitors; Nivolumab; PD-1; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, et al. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 2015;75:2200–2210. doi: 10.1158/0008-5472.CAN-14-2788. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

