Pembrolizumab induced pericardial tamponade: A case report
- PMID: 37143451
- PMCID: PMC10152068
- DOI: 10.1002/ccr3.7298
Pembrolizumab induced pericardial tamponade: A case report
Abstract
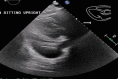
Key clinical message: The occurrence of a large pericardial effusion is not a commonly noted adverse event associated with pembrolizumab and our report demonstrates that a rapid development can be diagnosed with close monitoring and triage to acute medical settings.
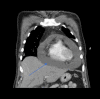
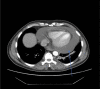
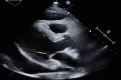
Abstract: Pembrolizumab is an immune checkpoint inhibitor used in various types of cancers. Pericardial tamponade is a rare side effect reported in only very few case reports. Early recognition and therapeutic intervention is vital in all cases. We report a case of a 54-year-old male with Stage 3 lung adenocarcinoma who developed cardiac tamponade secondary to pembrolizumab and subsequently required pericardial window.
Keywords: immunotherapy; lung adenocarcinoma; pembrolizumab; pericardial tamponade.
© 2023 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Flynn JP, Gerriets V. Pembrolizumab. StatPearls. StatPearls Publishing; 2022. - PubMed
-
- U.S. Food and Drug Administration . KEYTRUDA (pembrolizumab) reference ID: 4766009. Accessdata.Fda.Gov, https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
Publication types
LinkOut - more resources
Full Text Sources

