Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications
- PMID: 37143582
- PMCID: PMC10152985
- DOI: 10.1002/mco2.261
Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications
Abstract
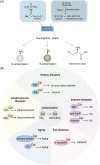
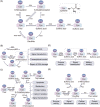
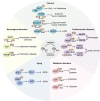
Protein posttranslational modifications (PTMs) refer to the breaking or generation of covalent bonds on the backbones or amino acid side chains of proteins and expand the diversity of proteins, which provides the basis for the emergence of organismal complexity. To date, more than 650 types of protein modifications, such as the most well-known phosphorylation, ubiquitination, glycosylation, methylation, SUMOylation, short-chain and long-chain acylation modifications, redox modifications, and irreversible modifications, have been described, and the inventory is still increasing. By changing the protein conformation, localization, activity, stability, charges, and interactions with other biomolecules, PTMs ultimately alter the phenotypes and biological processes of cells. The homeostasis of protein modifications is important to human health. Abnormal PTMs may cause changes in protein properties and loss of protein functions, which are closely related to the occurrence and development of various diseases. In this review, we systematically introduce the characteristics, regulatory mechanisms, and functions of various PTMs in health and diseases. In addition, the therapeutic prospects in various diseases by targeting PTMs and associated regulatory enzymes are also summarized. This work will deepen the understanding of protein modifications in health and diseases and promote the discovery of diagnostic and prognostic markers and drug targets for diseases.
Keywords: aging; cancers; metabolic diseases; neurodegenerative diseases; protein posttranslational modifications; targeted therapy.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Millan‐Zambrano G, Burton A, Bannister AJ, Schneider R. Histone post‐translational modifications: cause and consequence of genome function. Nat Rev Genet. 2022;23(9):563‐580. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
