Haemagglutination inhibition and virus microneutralisation serology assays: use of harmonised protocols and biological standards in seasonal influenza serology testing and their impact on inter-laboratory variation and assay correlation: A FLUCOP collaborative study
- PMID: 37143658
- PMCID: PMC10151801
- DOI: 10.3389/fimmu.2023.1155552
Haemagglutination inhibition and virus microneutralisation serology assays: use of harmonised protocols and biological standards in seasonal influenza serology testing and their impact on inter-laboratory variation and assay correlation: A FLUCOP collaborative study
Abstract
Introduction: The haemagglutination inhibition assay (HAI) and the virus microneutralisation assay (MN) are long-established methods for quantifying antibodies against influenza viruses. Despite their widespread use, both assays require standardisation to improve inter-laboratory agreement in testing. The FLUCOP consortium aims to develop a toolbox of standardised serology assays for seasonal influenza. Building upon previous collaborative studies to harmonise the HAI, in this study the FLUCOP consortium carried out a head-to-head comparison of harmonised HAI and MN protocols to better understand the relationship between HAI and MN titres, and the impact of assay harmonisation and standardisation on inter-laboratory variability and agreement between these methods.
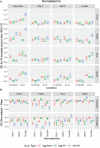
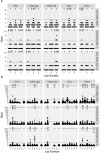
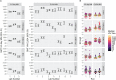
Methods: In this paper, we present two large international collaborative studies testing harmonised HAI and MN protocols across 10 participating laboratories. In the first, we expanded on previously published work, carrying out HAI testing using egg and cell isolated and propagated wild-type (WT) viruses in addition to high-growth reassortants typically used influenza vaccines strains using HAI. In the second we tested two MN protocols: an overnight ELISA-based format and a 3-5 day format, using reassortant viruses and a WT H3N2 cell isolated virus. As serum panels tested in both studies included many overlapping samples, we were able to look at the correlation of HAI and MN titres across different methods and for different influenza subtypes.
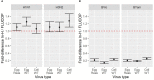
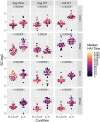
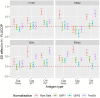
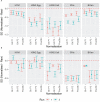
Results: We showed that the overnight ELISA and 3-5 day MN formats are not comparable, with titre ratios varying across the dynamic range of the assay. However, the ELISA MN and HAI are comparable, and a conversion factor could possibly be calculated. In both studies, the impact of normalising using a study standard was investigated, and we showed that for almost every strain and assay format tested, normalisation significantly reduced inter-laboratory variation, supporting the continued development of antibody standards for seasonal influenza viruses. Normalisation had no impact on the correlation between overnight ELISA and 3-5 day MN formats.
Keywords: haemagglutination inhibition assay (HAI); influenza viruses; serology; standardisation; virus microneutralisation assay (MN).
Copyright © 2023 Waldock, Remarque, Zheng, Ho, Hoschler, Neumann, Sediri-Schön, Trombetta, Montomoli, Marchi, Lapini, Zhou, Lartey, Cox, Facchini, Castrucci, Friel, Ollinger, Caillet, Music, Palladino, Engelhardt and the FLUCOP consortium.
Conflict of interest statement
LZ and CC are employed by Sanofi Pasteur. TO and DF are employees of the GSK group of companies. GP is employed by Seqirus. EM is Chief Scientific Officer of VisMederi srl and VisMederi Research srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- WHO . Influenza (Seasonal) (2018). Available at: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal).
-
- Waldock J, Zheng L, Remarque EJ, Civet A, Hu B, Jalloh SL, et al. . Assay harmonization and use of biological standards to improve the reproducibility of the hemagglutination inhibition assay: A flucop collaborative study. mSphere (2021) 6(4):e0056721. doi: 10.1128/mSphere.00567-21 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

