The generation, activation, and polarization of monocyte-derived macrophages in human malignancies
- PMID: 37143666
- PMCID: PMC10151765
- DOI: 10.3389/fimmu.2023.1178337
The generation, activation, and polarization of monocyte-derived macrophages in human malignancies
Abstract
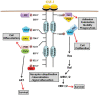
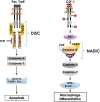
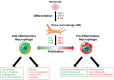
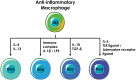
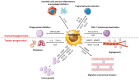
Macrophages are immune cells that originate from embryogenesis or from the differentiation of monocytes. They can adopt numerous phenotypes depending on their origin, tissue distribution and in response to different stimuli and tissue environment. Thus, in vivo, macrophages are endowed with a continuum of phenotypes that are rarely strictly pro-inflammatory or anti-inflammatory and exhibit a broad expression profile that sweeps over the whole polarization spectrum. Schematically, three main macrophage subpopulations coexist in human tissues: naïve macrophages also called M0, pro-inflammatory macrophages referred as M1 macrophages, and anti-inflammatory macrophages also known as M2 macrophages. Naïve macrophages display phagocytic functions, recognize pathogenic agents, and rapidly undergo polarization towards pro or anti-inflammatory macrophages to acquire their full panel of functions. Pro-inflammatory macrophages are widely involved in inflammatory response, during which they exert anti-microbial and anti-tumoral functions. By contrast, anti-inflammatory macrophages are implicated in the resolution of inflammation, the phagocytosis of cell debris and tissue reparation following injuries. Macrophages also play important deleterious or beneficial roles in the initiation and progression of different pathophysiological settings including solid and hematopoietic cancers. A better understanding of the molecular mechanisms involved in the generation, activation and polarization of macrophages is a prerequisite for the development of new therapeutic strategies to modulate macrophages functions in pathological situations.
Keywords: CSF-1; LAM; TAM; differentiation; monocyte-derived macrophages; polarization; targeting macrophages.
Copyright © 2023 Chaintreuil, Kerreneur, Bourgoin, Savy, Favreau, Robert, Jacquel and Auberger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

