12/15-lipoxygenase activity promotes efficient inflammation resolution in a murine model of Lyme arthritis
- PMID: 37143678
- PMCID: PMC10151577
- DOI: 10.3389/fimmu.2023.1144172
12/15-lipoxygenase activity promotes efficient inflammation resolution in a murine model of Lyme arthritis
Abstract
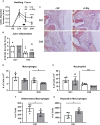
Infection of C3H/HeJ (C3H) mice with Borrelia burgdorferi results in the development of a robust inflammatory arthritis that peaks around 3-4 weeks post-infection and then spontaneously resolves over the next few weeks. Mice lacking cyclooxygenase (COX)-2 or 5-lipoxygenase (5-LO) activity develop arthritis similar to wild-type mice but display delayed or prolonged joint resolution. Since 12/15-lipoxygenase (12/15-LO) activity is generally down-stream of both COX-2 and 5-LO activity and results in the production of pro-resolution lipids such as lipoxins and resolvins among others, we investigated the impact of 12/15-LO deficiency on the resolution of Lyme arthritis in mice on a C3H background. We found the expression of Alox15 (12/15-LO gene) peaked around 4-weeks post-infection in C3H mice suggesting a role for 12/15-LO in mediating arthritis resolution. A deficiency in 12/15-LO resulted in exacerbated ankle swelling and arthritis severity during the resolution phase without compromising anti-Borrelia antibody production and spirochete clearance. However, clearance of inflammatory cells was impeded. Therapeutic treatment of B. burgdorferi-infected C3H mice with lipoxin A4 (LXA4) near the peak of disease resulted in significantly decreased ankle swelling and a switch of joint macrophages to a resolving phenotype but did not directly impact arthritis severity. These results demonstrate that 12/15-LO lipid metabolites are important components of inflammatory arthritis resolution in murine Lyme arthritis and may be a therapeutic target for treatment of joint edema and pain for Lyme arthritis patients without compromising spirochete clearance.
Keywords: Borrelia burgdorferi; Lyme disease; arthritis; eicosanoids; inflammation; resolution.
Copyright © 2023 Jackson, Hilliard and Brown.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

