Executive summary: Italian guidelines for diagnosis, risk stratification, and care continuity of fragility fractures 2021
- PMID: 37143730
- PMCID: PMC10151776
- DOI: 10.3389/fendo.2023.1137671
Executive summary: Italian guidelines for diagnosis, risk stratification, and care continuity of fragility fractures 2021
Abstract
Background: Fragility fractures are a major public health concern owing to their worrying and growing burden and their onerous burden upon health systems. There is now a substantial body of evidence that individuals who have already suffered a fragility fracture are at a greater risk for further fractures, thus suggesting the potential for secondary prevention in this field.
Purpose: This guideline aims to provide evidence-based recommendations for recognizing, stratifying the risk, treating, and managing patients with fragility fracture. This is a summary version of the full Italian guideline.
Methods: The Italian Fragility Fracture Team appointed by the Italian National Health Institute was employed from January 2020 to February 2021 to (i) identify previously published systematic reviews and guidelines on the field, (ii) formulate relevant clinical questions, (iii) systematically review literature and summarize evidence, (iv) draft the Evidence to Decision Framework, and (v) formulate recommendations.
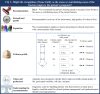
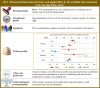
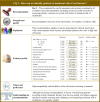
Results: Overall, 351 original papers were included in our systematic review to answer six clinical questions. Recommendations were categorized into issues concerning (i) frailty recognition as the cause of bone fracture, (ii) (re)fracture risk assessment, for prioritizing interventions, and (iii) treatment and management of patients experiencing fragility fractures. Six recommendations were overall developed, of which one, four, and one were of high, moderate, and low quality, respectively.
Conclusions: The current guidelines provide guidance to support individualized management of patients experiencing non-traumatic bone fracture to benefit from secondary prevention of (re)fracture. Although our recommendations are based on the best available evidence, questionable quality evidence is still available for some relevant clinical questions, so future research has the potential to reduce uncertainty about the effects of intervention and the reasons for doing so at a reasonable cost.
Keywords: evidence-based guideline; fragility fracture; grade; secondary prevention; systematic review.
Copyright © 2023 Corrao, Biffi, Porcu, Ronco, Adami, Alvaro, Bogini, Caputi, Cianferotti, Frediani, Gatti, Gonnelli, Iolascon, Lenzi, Leone, Michieli, Migliaccio, Nicoletti, Paoletta, Pennini, Piccirilli, Rossini, Tarantino and Brandi.
Conflict of interest statement
GC received research support from the European Community EC, the Italian Agency of Drug AIFA, and the Italian Ministry for University and Research MIUR. He took part to a variety of projects that were funded by pharmaceutical companies i.e., Novartis, GSK, Roche, AMGEN, and BMS. He also received honoraria as member of Advisory Board from Roche. No other potential conflicts of interest relevant to this article were disclosed. MLB has received i honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB; ii grants and/or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, UCB Pharma; and iii honoraria as consultant for Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, and UCB Pharma. LC has received honoraria as member of the Advisory Board from UCB Pharma and speaking fee of Dynamicom Education and took part to the Italian project for the introduction of Fracture Liaison Service. GA has received honoraria as consultant for Theramex. He took part to a project funded by the Italian Society of Rheumatology. DG has received honoraria as consultant for Eli-Lilly, Organon, and MSD Italia. SG has received honoraria as consultant for UCB Pharma. SM has received honoraria as consultant for UCB, Eli-Lilly, and Amgen. MR has received honoraria as consultant for UCB, Eli-Lilly, Theramex, and Amgen. He took part to a project funded by Savio Pharma Italia and UCB Pharma. RM took part to a project funded by Abiogen Pharma. GI received honoraria as speaker by Eli-Lilly, Menarini, and UCB Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- WHO. Report of a World Health Organization Study Group . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series, no. 843 (1994). Geneva. Available at: https://apps.who.int/iris/bitstream/handle/10665/39142/WHOTRS843eng.pdf?... (Accessed August 2022). - PubMed
-
- International Osteoporosis Foundation . Broken bones, broken lives – the fragility fracture crisis in six European countries (2018). Available at: https://www.iofbonehealth.org/broken-bones-broken-lives (Accessed August 2022).
-
- National Institute for Health and Care Excellence . Glossary . Available at: https://www.nice.org.uk/glossary (Accessed August 2022).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

