The Bronchiectasis Exacerbation Diary: a novel patient-reported outcome for non-cystic fibrosis bronchiectasis
- PMID: 37143836
- PMCID: PMC10152244
- DOI: 10.1183/23120541.00712-2022
The Bronchiectasis Exacerbation Diary: a novel patient-reported outcome for non-cystic fibrosis bronchiectasis
Abstract
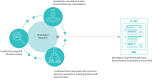
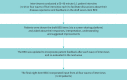
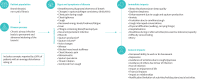
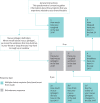
Bronchiectasis is a chronic, progressive lung disease believed to result from a vicious cycle of infection and inflammation, with symptoms of chronic cough with sputum production, chronic fatigue, rhinosinusitis, chest pain, breathlessness and haemoptysis. There are currently no established instruments to monitor daily symptoms and exacerbations for use in clinical trials. Following a literature review and three expert clinician interviews, we conducted concept elicitation interviews with 20 patients with bronchiectasis to understand their personal disease experience. Findings from literature and clinician feedback were used to develop a draft version of the Bronchiectasis Exacerbation Diary (BED), which was designed to monitor key symptoms on a daily basis and during exacerbations. Patients were eligible to be interviewed if they were US residents aged ≥18 years, had a computed tomography scan-confirmed diagnosis of bronchiectasis with ≥two exacerbations in the previous 2 years and had no other uncontrolled respiratory conditions. Four waves of five patient interviews each were conducted. Patients (n=20) had a mean±SD age of 53.9±12.8 years, and most were female (85%) and white (85%). A total of 33 symptoms and 23 impacts arose from the patient concept elicitation interviews. The BED was revised and finalised based upon patient feedback. The final BED is a novel, eight-item patient-reported outcome (PRO) instrument for monitoring key exacerbation symptoms on a daily basis with content validity established through comprehensive qualitative research and direct patient insight. The BED PRO development framework will be completed following psychometric evaluations of the data from a phase 3 bronchiectasis clinical trial.
Copyright ©The authors 2023.
Conflict of interest statement
Conflicts of interest: V.H. Shih, M. Jison and E. Bark are employees of AstraZeneca and may own stock. M. Venerus and O. Meyers are employees of IQVIA, which received funding from AstraZeneca to conduct this study. J.D. Chalmers has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Novartis, and Insmed, as well as consultancy or speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Insmed, Janssen, and Zambon.
Figures

References
LinkOut - more resources
Full Text Sources
