Parasagittal dural space hypertrophy and amyloid-β deposition in Alzheimer's disease
- PMID: 37143860
- PMCID: PMC10152899
- DOI: 10.1093/braincomms/fcad128
Parasagittal dural space hypertrophy and amyloid-β deposition in Alzheimer's disease
Abstract
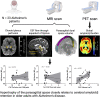
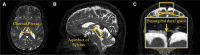
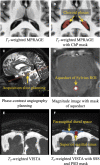
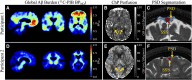
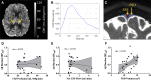
One of the pathological hallmarks of Alzheimer's and related diseases is the increased accumulation of protein amyloid-β in the brain parenchyma. As such, recent studies have focused on characterizing protein and related clearance pathways involving perivascular flow of neurofluids, but human studies of these pathways are limited owing to limited methods for evaluating neurofluid circulation non-invasively in vivo. Here, we utilize non-invasive MRI methods to explore surrogate measures of CSF production, bulk flow and egress in the context of independent PET measures of amyloid-β accumulation in older adults. Participants (N = 23) were scanned at 3.0 T with 3D T2-weighted turbo spin echo, 2D perfusion-weighted pseudo-continuous arterial spin labelling and phase-contrast angiography to quantify parasagittal dural space volume, choroid plexus perfusion and net CSF flow through the aqueduct of Sylvius, respectively. All participants also underwent dynamic PET imaging with amyloid-β tracer 11C-Pittsburgh Compound B to quantify global cerebral amyloid-β accumulation. Spearman's correlation analyses revealed a significant relationship between global amyloid-β accumulation and parasagittal dural space volume (rho = 0.529, P = 0.010), specifically in the frontal (rho = 0.527, P = 0.010) and parietal (rho = 0.616, P = 0.002) subsegments. No relationships were observed between amyloid-β and choroid plexus perfusion nor net CSF flow. Findings suggest that parasagittal dural space hypertrophy, and its possible role in CSF-mediated clearance, may be closely related to global amyloid-β accumulation. These findings are discussed in the context of our growing understanding of the physiological mechanisms of amyloid-β aggregation and clearance via neurofluids.
Keywords: amyloid-β; cerebrospinal fluid; choroid plexus; glymphatics; parasagittal dural space.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Klunk WE, Engler H, Nordberg A, et al. . Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
