CRISPR‑based diagnostic approaches: Implications for rapid management of future pandemics (Review)
- PMID: 37144477
- PMCID: PMC10196884
- DOI: 10.3892/mmr.2023.13005
CRISPR‑based diagnostic approaches: Implications for rapid management of future pandemics (Review)
Abstract
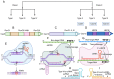
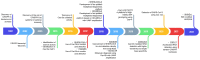
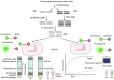
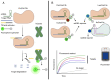
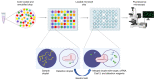
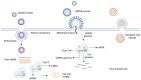
Sudden viral outbreaks have increased in the early part of the 21st century, such as those of severe acute respiratory syndrome coronavirus (SARS‑CoV), Middle East respiratory syndrome corona virus, and SARS‑CoV‑2, owing to increased human access to wildlife habitats. Therefore, the likelihood of zoonotic transmission of human‑associated viruses has increased. The emergence of severe acute respiratory syndrome coronavirus 2 in China and its spread worldwide within months have highlighted the need to be ready with advanced diagnostic and antiviral approaches to treat newly emerging diseases with minimal harm to human health. The gold‑standard molecular diagnostic approaches currently used are time‑consuming, require trained personnel and sophisticated equipment, and therefore cannot be used as point‑of‑care devices for widespread monitoring and surveillance. Clustered regularly interspaced short palindromic repeats (CRISPR)‑associated (Cas) systems are widespread and have been reported in bacteria, archaea and bacteriophages. CRISPR‑Cas systems are organized into CRISPR arrays and adjacent Cas proteins. The detection and in‑depth biochemical characterization of class 2 type V and VI CRISPR‑Cas systems and orthologous proteins such as Cas12 and Cas13 have led to the development of CRISPR‑based diagnostic approaches, which have been used to detect viral diseases and distinguish between serotypes and subtypes. CRISPR‑based diagnostic approaches detect human single nucleotide polymorphisms in samples from patients with cancer and are used as antiviral agents to detect and destroy viruses that contain RNA as a genome. CRISPR‑based diagnostic approaches are likely to improve disease detection methods in the 21st century owing to their ease of development, low cost, reduced turnaround time, multiplexing and ease of deployment. The present review discusses the biochemical properties of Cas12 and Cas13 orthologs in viral disease detection and other applications. The present review expands the scope of CRISPR‑based diagnostic approaches to detect diseases and fight viruses as antivirals.
Keywords: CRISPR; CRISPR RNA; CRISPR‑associated systems; collateral activity; diagnostics; fluorescence‑quencher; point‑of‑care devices; reporter; sensitivity; specificity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
CRISPR systems: Novel approaches for detection and combating COVID-19.Virus Res. 2021 Mar;294:198282. doi: 10.1016/j.virusres.2020.198282. Epub 2021 Jan 8. Virus Res. 2021. PMID: 33428981 Free PMC article. Review.
-
Development and application of sensitive, specific, and rapid CRISPR-Cas13-based diagnosis.J Med Virol. 2021 Jul;93(7):4198-4204. doi: 10.1002/jmv.26889. Epub 2021 Mar 25. J Med Virol. 2021. PMID: 33599292 Free PMC article. Review.
-
The era of Cas12 and Cas13 CRISPR-based disease diagnosis.Crit Rev Microbiol. 2022 Nov;48(6):714-729. doi: 10.1080/1040841X.2021.2025041. Epub 2022 Feb 15. Crit Rev Microbiol. 2022. PMID: 35164636
-
Applications of the CRISPR-Cas system for infectious disease diagnostics.Expert Rev Mol Diagn. 2021 Jul;21(7):723-732. doi: 10.1080/14737159.2021.1922080. Epub 2021 May 18. Expert Rev Mol Diagn. 2021. PMID: 33899643 Review.
-
CRISPR-based point-of-care diagnostics incorporating Cas9, Cas12, and Cas13 enzymes advanced for SARS-CoV-2 detection.J Biochem Mol Toxicol. 2022 Aug;36(8):e23113. doi: 10.1002/jbt.23113. Epub 2022 Jun 1. J Biochem Mol Toxicol. 2022. PMID: 35642647 Free PMC article. Review.
Cited by
-
Ultrasensitive CRISPR/Cas12a-Based System for Detection of BlaOXA-1 Gene in Antibiotic-Resistant Microorganisms.Curr Issues Mol Biol. 2025 Mar 29;47(4):238. doi: 10.3390/cimb47040238. Curr Issues Mol Biol. 2025. PMID: 40699637 Free PMC article.
-
Dengue and Flavivirus Co-Infections: Challenges in Diagnosis, Treatment, and Disease Management.Int J Mol Sci. 2025 Jul 10;26(14):6609. doi: 10.3390/ijms26146609. Int J Mol Sci. 2025. PMID: 40724859 Free PMC article. Review.
-
CRISPR-Cas12a/RPA Dual-Readout Assay for Rapid Field Detection of Porcine Rotavirus with Visualization.Viruses. 2025 Jun 20;17(7):872. doi: 10.3390/v17070872. Viruses. 2025. PMID: 40733490 Free PMC article.
References
-
- Jayamohan H, Lambert CJ, Sant HJ, Jafek A, Patel D, Feng H, Beeman M, Mahmood T, Nze U, Gale BK. SARS-CoV-2 pandemic: A review of molecular diagnostic tools including sample collection and commercial response with associated advantages and limitations. Anal Bioanal Chem. 2021;413:49–71. doi: 10.1007/s00216-020-02958-1. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

